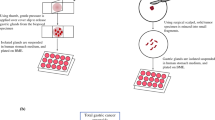
Abstract
Purpose
Colorectal endoscopic submucosal dissection (ESD) produces exfoliated tumor cells that occasionally cause local recurrence. However, the biological characteristics of these tumor cells have not been clarified. The aim of this study was to clarify the genetic background and viability of exfoliated tumor cells in colorectal ESDs, as well as possible method for their elimination.
Methods
Post-ESD intraluminal lavage samples from 19 patients who underwent colorectal ESDs were collected. In four patients with adenocarcinoma, gene mutations in the primary tumors and exfoliated cells in lavage samples were analyzed using a next-generation sequencer (NGS). In 15 patients with adenoma or adenocarcinoma, the viability of exfoliated cells and the cell-killing effect of povidone-iodine on exfoliated cells were evaluated.
Results
The analysis using a NGS demonstrated that tumors targeted for ESD had already acquired mutations in many genes involved in cell proliferation, angiogenesis, and invasions. Furthermore, gene mutations between the exfoliated tumor cells and tumors resected by ESDs showed a 92 to 100% concordance. The median viable cell counts and the median viability of exfoliated cells in intraluminal lavage samples after ESDs were 4.9 × 105 cells/mL and 24%, respectively. The viability of the exfoliated cells did not decrease even 12 h after ESD. However, contact with 2.0% povidone-iodine solution reduced both viable cell counts and viability, significantly.
Conclusion
A large number of tumor cells exfoliated during colorectal ESDs had acquired survival-favorable gene mutations and could survive for some time. Therefore, a lavage using a solution of 2.0% povidone-iodine may be effective against such cells.
Trial registration
The prospective study registered 1317, and the retrospective study registered 2729. The prospective study approved on June 20, 2016, and the retrospective study approved on October 6, 2020.




Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Dumoulin FL, Hildenbrand R (2019) Endoscopic resection techniques for colorectal neoplasia: current developments. World J Gastroenterol 25:300–307
Komeda Y, Watanabe T, Sakurai T et al (2019) Risk factors for local recurrence and appropriate surveillance interval after endoscopic resection. World J Gastroenterol 25:1502–1512
Tanaka S, Kashida H, Saito Y et al (2020) Japan Gastroenterological Endoscopy Society Guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 32:219–239
Tanaka S, Terasaki M, Kanao H et al (2012) Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig Endosc 24:73–79
Yoshida N, Yagi N, Naito Y et al (2010) Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol 16:1688–1695
Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T et al (2015) Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 47:829–854
Kaltenbach T, Anderson JC, Burke CA et al (2020) Endoscopic removal of colorectal lesions-recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc 158:1095–1129
Saito Y, Fukuzawa M, Matsuda T et al (2010) Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endos 24:343–352
Fujiya M, Tanaka K, Dakoshi T et al (2015) Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: A meta-analysis of studies comparing emr and endoscopic submucosal dissection. Gastrointest Endosc 81:583–595
Oka S, Tanaka S, Saito Y et al (2015) Local recurrence after endoscopic resection for large colorectal neoplasia: A multicenter prospective study in Japan. Am J Gastroenterol 110:697–707. https://doi.org/10.1038/ajg.2015.96
Suchy C, Berger M, Steinbrück I et al (2021) Long-term follow-up after colorectal endoscopic submucosal dissection in 182 cases. Endosc Int Open 9:E258–E262
Morgan CN (1951) Cancer of the rectum. Ann R Coll Surg Engl 9:13
Inoue T, Fujii H, Koyama F et al (2014) Local recurrence after rectal endoscopic submucosal dissection: A case of tumor cell implantation. Clin J Gastroenterol 7:36–40
Shinhata H, Yamamoto H, Sunada K et al (2015) Advanced rectal carcinoma caused by tumor cell implantation after curative endoscopic submucosal dissection of an intramucosal rectal carcinoma. Endoscopy 47:E192-194
Nakano Y, Toyonaga T, Nishino E et al (2019) Recurrence of adenoma after curative endoscopic submucosal dissection for a rectal intramucosal adenocarcinoma in adenoma. Endosc Int Open 07:E621-624
Inoue T, Fujii H, Koyama F et al (2014) Exfoliated Tumor Cells in Intraluminal Lavage Samples after Colorectal Endoscopic Submucosal Dissection: a Pilot Study. Hepatogastroenterology 61:667–670
Inoue T, Fujii H, Koyama F et al (2016) Intraluminal lavage to remove exfoliated tumor cells after colorectal endoscopic submucosal dissection. Surg Endosc 30:2773–2778
Watanabe T, Itabashi M, Shimada Y et al (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20:207–239
Kingsmore S. Catalogue of Somatic Mutations in Cancer – Home Page. http://cancer.sanger.ac.uk/cosmic. Accessed October 25, 2017.
Tennant JR (1964) Evaluation of the trypan blue technique for determination of cell viability. Transplantation 2:685–694. https://doi.org/10.1097/00007890-196411000-00001
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon (2003) November 30 to December 1, 2002. Gastrointest Endosc 58 6 Suppl S3-43. https://doi.org/10.1016/s0016-5107(03)02159-x
Kodeda K, Holmberg E, Jörgren F et al (2010) Rectal washout and local recurrence of cancer after anterior resection. Br J Surg 97:1589–1597
Maeda K, Maruta M, Hanai T et al (2004) Irrigation volume determines the efficacy of ‘rectal washout.’ Dis Colon Rectum 47:1706–1710. https://doi.org/10.1007/s10350-004-0659-z
Umpleby HC, Williamson RC (1984) The efficacy of agents employed to prevent anastomotic recurrence in colorectal carcinoma. Ann R Coll Surg Engl 66:192
Basha G, Penninckx F, Yap P (1998) Influence of blood components and faeces on the in vitro cancericidal activity of povidone-iodine. Br J Surg 85:534–537
Banich FE, Mendak SJ (1989) Intraoperative colonic irrigation with povidone iodine - An effective method of wound sepsis prevention. Dis Colon Rectum 32:219–222
Acknowledgements
We thank Mr. Naoto Kondo, Mr. Takanori Washio, and Ms. Emi Kanno (Riken Genesis Co., Ltd., Kawasaki, Japan) for their technical assistance in gene mutation sequencing using next-generation sequencing.
Funding
This study was supported in part by the Japan Society for the Promotion of Science KAKENHI Grant (No. 18K16326 to T. Inoue).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Takayuki Nakamoto, Fumikazu Koyama, Hiroyuki Kuge, Shinsaku Obara, Yosuke Iwasa, Takeshi Takei, Tomomi Sadamitsu, Suzuka Harada, Kosuke Fujimoto, Takashi Inoue. Histological examinations were performed by Kinta Hatakeyama and Chiho Ohbayashi. Data analysis and interpretation were performed by Takayuki Nakamoto, Fumikazu Koyama, Hiroyuki Kuge, Shinsaku Obara, Naoya Ikeda, Kinta Hatakeyama, Chiho Ohbayashi, and Masayuki Sho. The first draft of the manuscript was written by Takayuki Nakamoto, Fumikazu Koyama, and Naoya Ikeda, and critical revision of the manuscript was performed by Fumikazu Koyama, Takayuki Nakamoto and Masayuki Sho. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by our institutional ethics committee (No. 1317 and 2729). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all the patients.
Consent for publication
Written informed consent was obtained from all the patients.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakamoto, T., Koyama, F., Kuge, H. et al. In vitro analysis of exfoliated tumor cells in intraluminal lavage samples after colorectal endoscopic submucosal dissection. Int J Colorectal Dis 37, 161–170 (2022). https://doi.org/10.1007/s00384-021-04037-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-04037-y