Abstract
Purpose
Interleukin-6 (IL-6) production and signalling are increased in the inflamed mucosa in inflammatory bowel diseases (IBD). As published serum levels of IL-6 and its soluble receptors sIL-6R and sgp130 in IBD are from small cohorts and partly contradictory, we systematically evaluated IL-6, sIL-6R and sgp130 levels as markers of disease activity in Crohn’s disease (CD) and ulcerative colitis (UC).
Methods
Consecutive adult outpatients with confirmed CD or UC were included, and their disease activity and medication were monitored. Serum from 212 CD patients (815 measurements) and 166 UC patients (514 measurements) was analysed, and 100 age-matched healthy blood donors were used as controls.
Results
IL-6 serum levels were significantly elevated in active versus inactive CD and UC, also compared with healthy controls. However, only a fraction of IBD patients showed increased serum IL-6. IL-6 levels ranged up to 32.7 ng/mL in active CD (> 5000-fold higher than in controls), but also up to 6.9 ng/mL in inactive CD. Increases in active UC (up to 195 pg/mL) and inactive UC (up to 27 pg/mL) were less pronounced. Associations between IL-6 serum levels and C-reactive protein concentrations as well as leukocyte and thrombocyte counts were observed. Median sIL-6R and sgp130 levels were only increased by up to 15%, which was considered of no diagnostic significance.
Conclusions
Only a minority of IBD patients shows elevated IL-6 serum levels. However, in these patients, IL-6 is strongly associated with disease activity. Its soluble receptors sIL-6R and sgp130 do not appear useful as biomarkers in IBD.
Similar content being viewed by others
Introduction
The pleiotropic cytokine interleukin-6 (IL-6) plays an important role in inflammatory diseases and inflammatory carcinogenesis. Strategies for its inhibition have gained increasing interest due to the success of the anti-interleukin-6 receptor (IL-6R) antibody tocilizumab in rheumatologic diseases [1,2,3,4]. A significantly increased IL-6 production was reported in stimulated monocytes from patients with active inflammatory bowel disease (IBD) in comparison with samples from inactive disease phases or healthy control individuals [5, 6]. A general increase of IL-6 in patients with IBD and animal models has been reported in several studies [7,8,9,10,11,12,13].
IL-6 signals via two mechanisms, classic signalling and trans-signalling [1,2,3,4, 14]. In classic signalling, IL-6 binds to the specific cell membrane IL-6R expressed only on certain cell types, mainly leukocytes and hepatocytes, and the resulting IL-6/IL-6R complex recruits preformed dimers of the ubiquitously expressed IL-6 signal transducer glycoprotein 130 (gp130). In trans-signalling, IL-6 binds to soluble IL-6R (sIL-6R) formed by alternative splicing or, mainly, by proteolytic shedding of IL-6R from cell membranes. The resulting IL-6/sIL-6R trans-signalling complex acts like a different, more pro-inflammatory cytokine with a much broader target range, as it can theoretically activate gp130 signal transduction on every body cell, resulting in signalling patterns that are similar to but consequentially distinct from those of classic IL-6 signalling. In order to prevent such global trans-signalling, soluble forms of gp130 (sgp130) buffer the IL-6/sIL-6R complex in the blood. In the absence of inflammatory triggers, IL-6 is expressed in the low picogram range, whereas sIL-6R and sgp130 are expressed in the low to mid nanogram range (Supplemental Table 1). This mechanism indirectly also partly buffers IL-6 signalling as a whole [15], and even small congenital elevations of sIL-6R levels can reduce the risk for cardiovascular and autoimmune diseases [1, 4, 14].
There is good evidence that IL-6 levels, particularly in the context of IL-6 trans-signalling leading to activation of the pro-inflammatory signal transducer and activator of transcription-3 (STAT3), are strongly elevated in the inflamed mucosa in IBD [16,17,18,19,20,21,22,23]. IL-6 is the main inducer of C-reactive protein (CRP) [24], and IL-6 and sIL-6R levels have been shown by some studies to be positively associated with CRP levels in IBD [9, 11, 25]. A pilot phase II study using the neutralising anti-IL-6R antibody tocilizumab suggested a clinical response in Crohn’s disease (CD) patients [26], and several inhibitors of the IL-6 pathway are in clinical development, some also for IBD [3, 14, 27]. Multiple studies have reported significant increases in circulating IL-6 and sIL-6R in patients with IBD, albeit in different concentration ranges (Supplementary Table 1), as well as positive associations between IL-6 and sIL-6R serum levels and both activity indices and biomarkers of IBD [9, 11, 23, 25, 28, 29]. However, published levels and associations of biomarkers in IBD in general [30] and of IL-6, sIL-6R and sgp130 in particular are so diverse and partly contradictory (Supplementary Table 1) that a systematic analysis on a larger scale appeared warranted.
Therefore, the aim of the present study was to systematically analyse IL-6 and sIL-6R serum levels in a large all-comer cohort of outpatients with CD and ulcerative colitis (UC) and healthy control individuals. In addition, sgp130 levels were measured in a subset of patients and in all controls during the exploratory phase of the study. Multiple samplings and longitudinal follow-up in all available patients as well as a study duration of more than 1 year were used in this real-life, single-centre cohort to minimise bias and optimise association analyses with numerous physiological and pathophysiological parameters. Taken together, our study provides comprehensive data on the suitability and limitations of IL-6, sIL-6R and sgp130 as potential biomarkers in IBD.
Materials and methods
Patients and clinical data collection
In the IBD outpatient clinic of the Department of Internal Medicine I at the University Hospital Schleswig-Holstein (UKSH, Campus Kiel, Germany), consecutive adult patients with confirmed IBD were characterised and their disease activity as well as their medical treatment was monitored by regular visits, laboratory assessments of serum aliquots, clinical examinations, disease activity scores recorded at the time of blood collection (CD: Crohn’s Disease Activity Index (CDAI) [31] and Harvey-Bradshaw Index (HBI) [32]; UC: Colitis Activity Index (CAI) [33] and (Partial) Mayo Score [34]) as well as endoscopies. Diverse parameters were retrieved from the patients’ electronic hospital records and double-checked by at least two physicians. Serum from 100 age-matched healthy blood donors was obtained from the UKSH Department of Transfusion Medicine to serve as a control group. Ethics approval was granted by the ethics committee of the medical faculty of Kiel University prior to the study (AZ D489/14). The ethical concept of healthcare-embedded biobanking with broad informed consents as implemented in Kiel has recently been described [35]. Characteristics of IBD patients and healthy controls are summarised in Table 1.
Measurement of IL-6, sIL-6R and sgp130
IL-6 and sIL-6R levels were determined in all patients and control samples. In the exploratory phase of the study, sgp130 levels were also determined in all control samples and in 212 patients with CD and 166 patients with UC, the ratio approximately reflecting the proportions of outpatients in our clinic. Quantikine® enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems/Bio-Tecne; Wiesbaden, Germany) were used with a highly standardised protocol (for details, see Supplementary methods).
Data analysis and statistics
All parameters investigated in the present study are listed in Supplementary Table 2. For details of the statistical methods, see Supplementary methods. Since more than 50% of the IL-6 measurements were below the detection limit of 3.13 pg/mL, we divided all IL-6 values of the complete dataset into three categories defined as (1) below detection limit, (2) between detection limit and third quartile and (3) larger than third quartile. We compared the two extreme groups 1 and 3 in all our analyses in order to clearly differentiate between patients with high IL-6 concentrations and patients with signals close to the detection limit. Concentrations of IL-6, sIL-6R and sgp130 were compared within patients, between patient groups and between patients and healthy controls. For each patient, we defined active and/or inactive time points based on either disease activity scores (active: CDAI > 150 points or CAI > 4 points; elevated (moderate to severe) activity: CDAI > 250 points or CAI > 6 points; inactive: CDAI ≤ 150 points or CAI ≤ 4 points) or CRP levels (active: CRP > 5 mg/L; inactive: CRP ≤ 5 mg/L). We used two different cohorts in our comparisons. First, we used all active and inactive time points from all patients (full dataset/cohort), and second, we selected only the most active (highest CDAI, CAI or CRP) and the most inactive time points (lowest CDAI, CAI or CRP) from those patients for whom both active and inactive time points were available (reduced dataset/cohort). Supplementary Table 3 shows the number of patients and measurements for the cohorts and subcohorts. Comparisons between active, inactive and healthy control samples were performed using a nonparametric bootstrap approach. Associations with clinical and serological parameters were analysed using all available data. For data presented in boxplots, the box corresponds to the interquartile range, the median is displayed as a bold line, the whiskers represent the most extreme values within the 1.5-fold of the interquartile range and values outside this range are displayed as single dots.
Results
IL-6, sIL-6R and sgp130 levels in the complete patient cohorts
Table 2 summarises the measured serum levels of IL-6, sIL-6R and sgp130. Figure 1 shows the overall distribution of values. For the respective statistical data, see Supplementary Tables 4 and 5. As > 50% of IL-6 measurements in all groups were below the detection limit (≤ 3.13 pg/mL), ordinal IL-6 data were statistically evaluated by comparing the two most extreme groups (below detection limit vs top quartile). Figure 1a shows that the proportion of values above the detection limit is increased in IBD compared to healthy controls. Quantitative IL-6 data are presented on a logarithmic scale due to their extreme variation (Fig. 1b). While an increase in variation compared to normal controls was also observed in CD and UC patients for both sIL-6R (Fig. 1c) and sgp130 (Fig. 1d), the increase of their median levels was less than 15% and not consistent with regard to disease activity (Fig. 1c, d; Table 2).
Overview of IL-6, sIL-6R and sgp130 serum levels in healthy controls and patients with Crohn’s disease (CD) or ulcerative colitis (UC). a Ordinal representation of IL-6 levels across all groups: below the detection limit of 3.13 pg/mL (light grey), between the detection limit and the third quartile (grey) and above the third quartile (black). b Logarithmic representation of IL-6 levels. c sIL-6R levels. d sgp130 levels
When patient subgroups were analysed based on their disease activity scores (Fig. 2) or CRP levels (Fig. 3), elevated IL-6 levels were significantly associated with disease activity in both CD and UC. For the respective statistical data, see Supplementary Tables 6 and 7. This association was more pronounced with CRP than with disease activity scores, particularly in UC (cf. Figs. 2a and 3a). A subgroup of patients with elevated (moderate to severe) disease activity scores (“ea” in Fig. 2; CDAI > 250 or CAI > 6) showed higher IL-6 levels than all active patients, including those with mild activity, taken together (“a” in Fig. 2; CDAI > 150 or CAI > 4). Nevertheless, the majority also of patients with active CD or UC did not show elevated IL-6 levels (Table 2; Fig. 2a). The minor increase in sIL-6R levels was independent of disease activity in both CD and UC (Figs. 2c and 3c) and, as with IL-6, was more pronounced when patients were grouped according to CRP levels (Fig. 3c).
IL-6, sIL-6R and sgp130 serum levels in healthy controls and in all patients with Crohn’s disease (CD) or ulcerative colitis (UC) differentiated according to disease activity scores. a Ordinal representation of IL-6 levels across all groups: below the detection limit of 3.13 pg/mL (light grey), between the detection limit and the third quartile (grey) and above the third quartile (black). For significance analyses, the two extreme groups (below the detection limit and above the third quartile) were compared. b Logarithmic representation of IL-6 levels. c sIL-6R levels. d sgp130 levels. a, active disease (Crohn’s Disease Activity Index (CDAI) > 150 or Colitis Activity Index (CAI) > 4); ea, elevated (moderate to severe) disease activity (CDAI > 250 or CAI > 6); i, inactive disease (CDAI ≤ 150 or CAI ≤ 4). Significant differences between active and inactive disease: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant differences between active or inactive disease and controls: #, p < 0.05; ##, p < 0.01; ###, p < 0.001
IL-6, sIL-6R and sgp130 serum levels in healthy controls and in all patients with Crohn’s disease (CD) or ulcerative colitis (UC) differentiated according to C-reactive protein (CRP) levels. a Ordinal representation of IL-6 levels across all groups: below the detection limit of 3.13 pg/mL (light grey), between the detection limit and the third quartile (grey) and above the third quartile (black). For significance analyses, the two extreme groups (below the detection limit and above the third quartile) were compared. b Logarithmic representation of IL-6 levels. c sIL-6R levels. d sgp130 levels. a, active disease (CRP > 5 mg/L); i, inactive disease (CRP ≤ 5 mg/L). Significant differences between active and inactive disease: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant differences between active or inactive disease and controls: #, p < 0.05; ##, p < 0.01; ###, p < 0.001
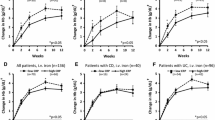
IL-6, sIL-6R and sgp130 levels in subcohorts with matched measurements
In order to investigate whether the large number of patients with inactive disease and the multiple visits per patient (in which disease activity was eventually controlled by anti-inflammatory drugs) confounded conclusions drawn from the complete dataset, a reduced dataset for patients with CD and UC was analysed (Supplementary Table 3). For this purpose, only patients with at least one time point with active and one time point with inactive disease were included. If more than one time point per condition was available, the most contrasting data (highest and lowest disease activity scores (Fig. 4) or CRP levels (Fig. 5)) were analysed. For the respective statistical data, see Supplementary Tables 8 and 9. The results of this subcohort analysis did not differ much from the results obtained with the full dataset (Figs. 4 and 5; cf. Figs. 2 and 3). While significantly higher IL-6 serum levels were observed in active versus inactive disease, there were still many patients with active disease and normal IL-6 levels, regardless of whether activity was defined by disease score or CRP. Also in this subcohort, sIL-6R and gp130 levels were only slightly increased.
IL-6, sIL-6R and sgp130 serum levels in healthy controls and in selected patients with Crohn’s disease (CD) or ulcerative colitis (UC) differentiated according to disease activity scores. Only patients with at least one time point with active and one with inactive disease were included. If more than one time point per condition was available, only data from the two most extreme time points (highest and lowest disease activity score) were included. a Ordinal representation of IL-6 levels across all groups: below the detection limit of 3.13 pg/mL (light grey), between the detection limit and the third quartile (grey) and above the third quartile (black). For significance analyses, the two extreme groups (below the detection limit and above the third quartile) were compared. b Logarithmic representation of IL-6 levels. c sIL-6R levels. d sgp130 levels. a, active disease (Crohn’s Disease Activity Index (CDAI) > 150 or Colitis Activity Index (CAI) > 4); i, inactive disease (CDAI ≤ 150 or CAI ≤ 4). Significant differences between active and inactive disease: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant differences between active or inactive disease and controls: #, p < 0.05; ##, p < 0.01; ###, p < 0.001
IL-6, sIL-6R and sgp130 serum levels in healthy controls and in selected patients with Crohn’s disease (CD) or ulcerative colitis (UC) differentiated according to C-reactive protein (CRP) levels. Only patients with at least one time point with CRP > 5 mg/L and one time point with CRP ≤ 5 mg/L were included. If more than one time point per condition was available, only data from the two most extreme time points (highest and lowest CRP levels) were included. a Ordinal representation of IL-6 levels across all groups: below the detection limit of 3.13 pg/mL (light grey), between the detection limit and the third quartile (grey) and above the third quartile (black). For significance analyses, the two extreme groups (below the detection limit and above the third quartile) were compared. b Logarithmic representation of IL-6 levels. c sIL-6R levels. d sgp130 levels. a, active disease (CRP > 5 mg/L); i, inactive disease (CRP ≤ 5 mg/L). Significant differences between active and inactive disease: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant differences between active or inactive disease and controls: #, p < 0.05; ##, p < 0.01; ###, p < 0.001
Taken together, a consistent, both biologically and statistically significant, increase was observed only for IL-6, but not for sIL-6R or sgp130. However, while this increase in IL-6 was seen on the population level, it was less than 50% of individuals with active disease according to scores who contributed to the signal (Figs. 2a and 4a). In contrast, a majority of CD and UC patients with elevated CRP levels showed IL-6 levels above the detection limit (Figs. 3a and 5a).
Association of IL-6, sIL-6R and sgp130 levels with general, clinical and laboratory parameters
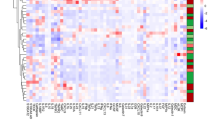
A wide spectrum of parameters was analysed for associations (Fig. 6; Supplementary Tables 2, 10, 11, 12 and 13). Disease activity-associated laboratory parameters were consistently positively (CRP, leukocytes and thrombocytes) or negatively (haematocrit and haemoglobin) associated with IL-6 levels in both CD and UC (Fig. 6a, b; Supplementary Table 10). There was no clear association between IL-6 and sIL-6R or sgp130 (Fig. 6a, b; Supplementary Tables 10, 11 and 12), but the slightly increased IL-6 buffer consisting of sIL-6R and sgp130 in CD and UC (Figs. 1, 2, 3, 4 and 5; Table 2) was mirrored by significant positive associations between these soluble receptor components (Fig. 6a, b; Supplementary Tables 11 and 12). In CD, but not in UC, sIL-6R and sgp130 levels were also positively associated with previous surgery on the gastrointestinal tract and suggestively (sIL-6R) or significantly (sgp130) negatively associated with haematocrit (Fig. 6a, b; Supplementary Tables 11 and 12). Regarding disease location, IL-6 was only positively associated with terminal ileitis in CD, while sIL-6R and sgp130 showed a complex association with several locations, but rather small effect sizes (Supplementary Table 13). In UC, no significant associations with disease location were observed (Supplementary Table 13). The use of tumour necrosis factor-alpha (TNF-α) antagonists was significantly associated with reduced IL-6 levels in CD (Fig. 6a), whereas the same was observed for aminosalicylates in UC (Fig. 6b).
Association heat maps for Crohn’s disease (CD; a) and ulcerative colitis (UC; b). Tendentially or significantly positive (increase) or negative (decrease) associations of ordinal interleukin-6, soluble interleukin-6 receptor or soluble glycoprotein 130 serum levels with other parameters are visualised by colour code. For the complete data and p values, see Supplementary Tables 10, 11 and 12. Grey fields indicate parameters with only one characteristic value or self-associations
Discussion
The main objective of the present study was to explore IL-6, sIL-6R and sgp130 as potential biomarkers in IBD on the background of conflicting prior reports (Supplemental Table 1) [7,8,9,10,11,12,13]. Previous publications demonstrating an overall increase in IL-6 levels in patients with IBD, particularly in those with active disease [7,8,9,10,11, 13], were confirmed by our findings. Broken down to the individual level, the signal was carried by less than 50% of patients with active disease: in contrast to previous smaller studies [7, 10, 11], median IL-6 levels were neither elevated in inactive nor in active disease (based on disease scores) compared to healthy control individuals, and the range of IL-6 levels was much wider [7, 10, 11]. Disease activity based on CRP was more strongly matched to elevated IL-6 levels than score-based activity, which is plausible due to the key role of IL-6 in CRP secretion.
Serum levels of IL-6 do not necessarily reflect the situation in the intestinal mucosa, where high local levels of IL-6 have been detected during inflammation [16,17,18,19,20,21,22,23], because blood circulation from the intestine through the liver may clear large amounts of IL-6. Similar to IL-6 in the present study, TNF-α serum levels are also not consistently found to be elevated in patients with IBD [36,37,38,39,40], despite the proven strong increase in mucosal TNF-α-producing cells and TNF-α levels in stool [16, 41,42,43,44,45] and the well-established efficacy of anti-TNF-α antibodies in CD and UC [46].
sIL-6R levels were only slightly increased in IBD patients. Our data confirm the findings of the small pilot study of Gustot et al. [11] and the general consensus on sIL-6R levels in health and chronic inflammation in the literature [1], whereas Nancey et al. [13] and particularly Mitsuyama et al. [9] measured higher overall levels. A tendency towards higher serum levels of sgp130 was observed in the present study for both active CD and UC, but no significant difference was observed between active and inactive patients. Again, our data confirm those by Gustot et al. [11] and the general consensus on sgp130 levels [1], whereas much less sgp130 was detected by Nancey et al. [13]. The reasons for the variance between these reports remain unclear, but the biological significance of both sIL-6R and sgp130 level changes appears to be limited in IBD.
We observed associations of IL-6, sIL-6R and sgp130 serum levels with general, clinical and laboratory parameters that largely confirm previous reports and are plausible with regard to the course of the diseases, including a positive association of IL-6 levels with disease activity, CRP levels as well as leukocyte and platelet counts. The use of TNF-α antagonists in CD and the use of aminosalicylates in UC were therapies for which we detected a significant reduction of IL-6 levels. The relevance of other associations is unclear: for example, the significant negative association between serum levels of the liver enzymes AST and ALT and the circulating components of the IL-6 system may reflect the abnormal liver function tests reported in IBD [47,48,49,50,51,52,53]. Some statistically significant associations also do not appear relevant due to their rather small effect sizes, such as the associations with disease location.
In summary, the present study shows that sIL-6R or sgp130 are not useful as biomarkers in IBD and that only a fraction of patients with active CD or UC have elevated IL-6 serum levels. It remains unexplored whether the individual differences reflect local IL-6 levels in the inflamed mucosa, too, or whether these are increased in the vast majority of patients. As a general biomarker of IBD disease activity, IL-6 adds little to established biomarkers like CRP, which, however, also have significant limitations. Taken together, IL-6 serum levels are another imperfect biomarker for IBD activity but may be useful for identifying patients with a particularly strong IL-6 disease signature in order to increase the likelihood of treatment success with drugs targeting IL-6 or its signalling pathways.
References
Scheller J, Garbers C, Rose-John S (2014) Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol 26:2–12
Waldner MJ, Neurath MF (2014) Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin Immunol 26:75–79
Hunter CA, Jones SA (2015) IL-6 as a keystone cytokine in health and disease. Nat Immunol 16:448–457
Schaper F, Rose-John S (2015) Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 26:475–487
Suzuki Y, Saito H, Kasanuki J, Kishimoto T, Tamura Y, Yoshida S (1990) Significant increase of interleukin 6 production in blood mononuclear leukocytes obtained from patients with active inflammatory bowel disease. Life Sci 47:2193–2197
Andus T, Gross V, Casar I, Krumm D, Hosp J, David M, Scholmerich J (1991) Activation of monocytes during inflammatory bowel disease. Pathobiology 59:166–170
Mahida YR, Kurlac L, Gallagher A, Hawkey CJ (1991) High circulating concentrations of interleukin-6 in active Crohn’s disease but not ulcerative colitis. Gut 32:1531–1534
Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL (1993) Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology 104:1285–1292
Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, Harada K, Tateishi H, Nishiyama T, Tanikawa K (1995) Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut 36:45–49
Reinisch W, Gasche C, Tillinger W, Wyatt J, Lichtenberger C, Willheim M, Dejaco C, Waldhor T, Bakos S, Vogelsang H, Gangl A, Lochs H (1999) Clinical relevance of serum interleukin-6 in Crohn’s disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol 94:2156–2164
Gustot T, Lemmers A, Louis E, Nicaise C, Quertinmont E, Belaiche J, Roland S, Van Gossum A, Deviere J, Franchimont D (2005) Profile of soluble cytokine receptors in Crohn’s disease. Gut 54:488–495
Mitsuyama K, Tomiyasu N, Suzuki A, Takaki K, Takedatsu H, Masuda J, Yamasaki H, Matsumoto S, Tsuruta O, Toyonaga A, Sata M (2006) A form of circulating interleukin-6 receptor component soluble gp130 as a potential interleukin-6 inhibitor in inflammatory bowel disease. Clin Exp Immunol 143:125–131
Nancey S, Hamzaoui N, Moussata D, Graber I, Bienvenu J, Flourie B (2008) Serum interleukin-6, soluble interleukin-6 receptor and Crohn’s disease activity. Dig Dis Sci 53:242–247
Garbers C, Aparicio-Siegmund S, Rose-John S (2015) The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol 34:75–82
Garbers C, Thaiss W, Jones GW, Waetzig GH, Lorenzen I, Guilhot F, Lissilaa R, Ferlin WG, Grotzinger J, Jones SA, Rose-John S, Scheller J (2011) Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J Biol Chem 286:42959–42970
Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A (1993) Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol 94:174–181
Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF (2000) Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med 6:583–588
Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, Eriksen KW, Woetmann A, Odum N (2003) Constitutive STAT3 activation in intestinal T cells from patients with Crohn’s disease. J Biol Chem 278:16777–16781
Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, Lehr HA, Schmidt J, Neurath MF (2005) Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol 100:64–72
Musso A, Dentelli P, Carlino A, Chiusa L, Repici A, Sturm A, Fiocchi C, Rizzetto M, Pegoraro L, Sategna-Guidetti C, Brizzi MF (2005) Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis 11:91–98
Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, Xia B, Kuipers EJ, van der Woude CJ (2010) Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 59:227–235
Neufert C, Pickert G, Zheng Y, Wittkopf N, Warntjen M, Nikolaev A, Ouyang W, Neurath MF, Becker C (2010) Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle 9:652–655
Bernardo D, Vallejo-Diez S, Mann ER, Al-Hassi HO, Martinez-Abad B, Montalvillo E, Tee CT, Murugananthan AU, Nunez H, Peake ST, Hart AL, Fernandez-Salazar L, Garrote JA, Arranz E, Knight SC (2012) IL-6 promotes immune responses in human ulcerative colitis and induces a skin-homing phenotype in the dendritic cells and T cells they stimulate. Eur J Immunol 42:1337–1353
Ganter U, Arcone R, Toniatti C, Morrone G, Ciliberto G (1989) Dual control of C-reactive protein gene expression by interleukin-1 and interleukin-6. EMBO J 8:3773–3779
Wine E, Mack DR, Hyams J, Otley AR, Markowitz J, Crandall WV, Leleiko N, Muise AM, Griffiths AM, Turner D (2013) Interleukin-6 is associated with steroid resistance and reflects disease activity in severe pediatric ulcerative colitis. J Crohns Colitis 7:916–922
Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N, Yoshizaki K, Shimoyama T, Kishimoto T (2004) A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 126:989–996
Danese S, Vermeire S, Hellstern P, Panaccione R, Rogler G, Fraser G, Kohn A, Desreumaux P, Leong RW, Comer GM, Cataldi F, Banerjee A, Maguire MK, Li C, Rath N, Beebe J, Schreiber S (2016) OP015 results of ANDANTE, a randomised clinical study with an anti-IL6 antibody (PF-04236921) in subjects with Crohn’s disease who are anti-tumour necrosis factor inadequate responders. J Crohn’s Colitis 10(suppl. 1):S12–S13
Louis E, Belaiche J, van Kemseke C, Franchimont D, de Groote D, Gueenen V, Mary JY (1997) A high serum concentration of interleukin-6 is predictive of relapse in quiescent Crohn’s disease. Eur J Gastroenterol Hepatol 9:939–944
Ljuca F, Gegic A, Salkic NN, Pavlovic-Calic N (2010) Circulating cytokines reflect mucosal inflammatory status in patients with Crohn’s disease. Dig Dis Sci 55:2316–2326
Faubion WA Jr, Fletcher JG, O’Byrne S, Feagan BG, de Villiers WJ, Salzberg B, Plevy S, Proctor DD, Valentine JF, Higgins PD, Harris JM, Diehl L, Wright L, Tew GW, Luca D, Basu K, Keir ME (2013) EMerging BiomARKers in Inflammatory Bowel Disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am J Gastroenterol 108:1891–1900
Best WR, Becktel JM, Singleton JW, Kern F Jr (1976) Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 70:439–444
Harvey RF, Bradshaw JM (1980) A simple index of Crohn’s-disease activity. Lancet 1:514
Rachmilewitz D (1989) Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 298:82–86
Schroeder KW, Tremaine WJ, Ilstrup DM (1987) Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 317:1625–1629
Richter G, Krawczak M, Lieb W, Wolff L, Schreiber S, Buyx A (2018) Broad consent for health care-embedded biobanking: understanding and reasons to donate in a large patient sample. Genet Med 20:76–82
Hyams JS, Treem WR, Eddy E, Wyzga N, Moore RE (1991) Tumor necrosis factor-alpha is not elevated in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 12:233–236
Murch SH, Lamkin VA, Savage MO, Walker-Smith JA, MacDonald TT (1991) Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut 32:913–917
Maeda M, Watanabe N, Neda H, Yamauchi N, Okamoto T, Sasaki H, Tsuji Y, Akiyama S, Tsuji N, Niitsu Y (1992) Serum tumor necrosis factor activity in inflammatory bowel disease. Immunopharmacol Immunotoxicol 14:451–461
Komatsu M, Kobayashi D, Saito K, Furuya D, Yagihashi A, Araake H, Tsuji N, Sakamaki S, Niitsu Y, Watanabe N (2001) Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem 47:1297–1301
Avdagic N, Babic N, Seremet M, Delic-Sarac M, Drace Z, Denjalic A, Nakas-Icindic E (2013) Tumor necrosis factor-alpha serum level in assessment of disease activity in inflammatory bowel diseases. Med Glas (Zenica) 10:211–216
MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A (1990) Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 81:301–305
Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT (1992) Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 339:89–91
Nicholls S, Stephens S, Braegger CP, Walker-Smith JA, MacDonald TT (1993) Cytokines in stools of children with inflammatory bowel disease or infective diarrhoea. J Clin Pathol 46:757–760
Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT (1994) Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 106:1455–1466
Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N (2002) Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol 97:2820–2828
Billmeier U, Dieterich W, Neurath MF, Atreya R (2016) Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol 22:9300–9313
Hyams J, Markowitz J, Treem W, Davis P, Grancher K, Daum F (1995) Characterization of hepatic abnormalities in children with inflammatory bowel disease. Inflamm Bowel Dis 1:27–33
Vadstrup S (2004) Subnormal alanine aminotransferase values in blood of patients with Crohn disease. Scand J Gastroenterol 39:554–556
Cappello M, Randazzo C, Bravata I, Licata A, Peralta S, Craxi A, Almasio PL (2014) Liver function test abnormalities in patients with inflammatory bowel diseases: a hospital-based survey. Clin Med Insights Gastroenterol 7:25–31
Goyal A, Hyams JS, Lerer T, Leleiko NS, Otley AR, Griffiths AM, Rosh JR, Cabrera JM, Oliva-Hemker MM, Mack DR, Rick JN, Pfefferkorn MD, Carvalho R, Grossman AB, Hitch MC, Sudel B, Kappelman MD, Saeed SA, Faubion WA, Schaefer ME, Markowitz JF, Keljo DJ (2014) Liver enzyme elevations within 3 months of diagnosis of inflammatory bowel disease and likelihood of liver disease. J Pediatr Gastroenterol Nutr 59:321–323
Pusateri AJ, Kim SC, Dotson JL, Balint JP, Potter CJ, Boyle BM, Crandall WV (2015) Incidence, pattern, and etiology of elevated liver enzymes in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 60:592–597
Schroder T, Schmidt KJ, Olsen V, Moller S, Mackenroth T, Sina C, Lehnert H, Fellermann K, Buning J (2015) Liver steatosis is a risk factor for hepatotoxicity in patients with inflammatory bowel disease under immunosuppressive treatment. Eur J Gastroenterol Hepatol 27:698–704
Carr RM, Patel A, Bownik H, Oranu A, Kerner C, Praestgaard A, Forde KA, Reddy KR, Lichtenstein GR (2017) Intestinal inflammation does not predict nonalcoholic fatty liver disease severity in inflammatory bowel disease patients. Dig Dis Sci 62:1354–1361
Acknowledgments
The authors thank Nadja Karl, Christin Löhner and Anja Schulze for excellent technical support.
Funding
This work was supported by Ferring Pharmaceuticals A/S (Copenhagen, Denmark), CONARIS Research Institute AG (Kiel, Germany), the German Excellence Cluster “Inflammation at Interfaces” (grant no. ExC306 to PR and SSc), the SysINFLAME consortium of the German Ministry of Education and Research (grant no. 01ZX1306 to PR and SSc), the collaborative research centre CRC1182 of the German Research Foundation (grant no. C2 to PR) and the SYSCID consortium of the EU Horizon 2020 programme (grant no. 733100 to PR and SSc).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GHW and DS are employed by CONARIS Research Institute AG, which has out-licenced its interleukin-6 inhibitor olamkicept to Ferring Pharmaceuticals. UL, BBR and TMR are employed by Ferring Pharmaceuticals. SSc heads the supervisory board of CONARIS. The other authors declare no conflict of interest.
Ethical approval
Ethics approval was granted by the ethics committee of the medical faculty of Kiel University prior to the study (AZ D489/14). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. The ethical concept of healthcare-embedded biobanking with broad informed consents as implemented in Kiel has recently been described [35].
Electronic supplementary material
ESM 1
(PDF 391 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nikolaus, S., Waetzig, G.H., Butzin, S. et al. Evaluation of interleukin-6 and its soluble receptor components sIL-6R and sgp130 as markers of inflammation in inflammatory bowel diseases. Int J Colorectal Dis 33, 927–936 (2018). https://doi.org/10.1007/s00384-018-3069-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-018-3069-8