Abstract
Purpose
Over the last few decades, several articles have examined the feasibility of attempting primary reduction and closure of gastroschisis without general anesthesia (GA). We aimed to systematically evaluate the impact of forgoing routine intubation and GA during primary bedside reduction and closure of gastroschisis.
Methods
The primary outcome was closure success. Secondary outcomes were mortality, time to enteral feeding, and length of hospital stay.
Results
12 studies were included: 5 comparative studies totalling 192 patients and 7 descriptive case studies totalling 56 patients. Primary closure success was statistically equivalent between the two groups, but trended toward improved success with GA/intubation (RR = 0.86, CI 0.70–1.03, p = 0.08). Mortality was equivalent between groups (RR = 1.26, CI 0.26–6.08, p = 0.65). With respect to time to enteral feeds and length of hospital stay, outcomes were either equivalent between the two groups or favored the group that underwent primary closure without intubation and GA.
Conclusion
There are few comparative studies examining the impact of performing primary bedside closure of gastroschisis without GA. A meta-analysis of the available data found no statistically significant difference when forgoing intubation and GA. Foregoing GA also did not negatively impact time to enteral feeds, length of hospital stay, or mortality.


Similar content being viewed by others
Availability of data, code, and other materials
Data and other materials can be obtained by contacting the corresponding author.
Abbreviations
- GA:
-
General anesthesia
- EI:
-
Endotracheal intubation
- ACS:
-
Abdominal compartment syndrome
- MINOR:
-
Methodological Index for Non-Randomized Studies
- NICU:
-
Neonatal Intensive Care Unit
References
Skarsgard ED (2016) Management of gastroschisis. Curr Opin Pediatr 28(3):363–369
Ledbetter DJ (2012) Congenital abdominal wall defects and reconstruction in pediatric surgery: gastroschisis and omphalocele. Surg Clin N Am 92(3):713–727
Kilby MD (2006) The incidence of gastroschisis. BMJ 332(7536):250–251
Stallings EB, Isenburg JL, Short TD et al (2019) Population-based birth defects data in the United States, 2012–2016: A focus on abdominal wall defects. Birth Defects Res 111(18):1436–1447
Fullerton BS, Velazco CS, Sparks EA et al (2017) Contemporary outcomes of infants with gastroschisis in North America: a multicenter cohort study. J Pediatr 188:192–197
Olesevich M, Alexander F, Khan M et al (2005) Gastroschisis revisited: role of intraoperative measurement of abdominal pressure. J Pediatr Surg 40(5):789–792
Kunz SN, Tieder JS, Whitlock K et al (2013) Primary fascial closure versus staged closure with silo in patients with gastroschisis: a meta-analysis. J Pediatr Surg 48(4):845–857
Filston HC (1983) Gastroschisis–primary fascial closure. The goal for optimal management. Ann Surg 197(3):260–264
Bowen J, Wilcox D, Bianchi A et al (1996) The umbilicus in gastroschisis: aesthetic considerations. Pediatr Surg Int 11(4):237–239
Bianchi A, Dickson AP (1998) Elective delayed reduction and no anesthesia: “minimal intervention management” for gastrochisis. J Pediatr Surg 33(9):1338–1340
Jevtovic-Todorovic V, Hartman RE, Izumi Y et al (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23(3):876–882
Vutskits L, Davidson A (2017) Update on developmental anesthesia neurotoxicity. Curr Opin Anaesthesiol 30(3):337–342
Raper J, Alvarado MC, Murphy KL et al (2015) Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology 123(5):1084–1092
Lee JH, Zhang J, Wei L et al (2015) Neurodevelopmental implications of the general anesthesia in neonate and infants. Exp Neurol 272:50–60
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73(9):712–716
Review Manager (RevMan) (Computer program) (2020) 5.4 ed: The Cochrane Collaboration
Higgins JPT TJ, Chandler J, Cumpston M, et al (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.2. In: Cochrane, editor. updated February 2021
Vane DW, Abajian JC, Hong AR (1994) Spinal anesthesia for primary repair of gastroschisis: a new and safe technique for selected patients. J Pediatr Surg 29(9):1234–1235
Dolgin SE, Midulla P, Shlasko E (2000) Unsatisfactory experience with “minimal intervention management” for gastroschisis. J Pediatr Surg 35(10):1437–1439
Kimble RM, Singh SJ, Bourke C, Cass DT (2001) Gastroschisis reduction under analgesia in the neonatal unit. J Pediatr Surg 36(11):1672–1674
Duncan ND, Brown B, Dundas SE, Wierenga K, Kulkarni S, Pinnock-Ramsaran C et al (2005) “Minimal intervention management” for gastroschisis: a preliminary report. West Indian Med J 54(2):152–154
Gore M, Joshi K, Dave N (2009) Combined spinal epidural anaesthesia for gastroschisis repair. Indian J Anaesth 53(2):223–225
Kasat N, Dave N, Shah H, Mahajan S (2017) Gastroschisis repair under caudal anesthesia: a series of three cases. Rev Bras Anestesiol 67(3):326–328
Davies MW, Kimble RM, Cartwright DW (2005) Gastroschisis: ward reduction compared with traditional reduction under general anesthesia. J Pediatr Surg 40(3):523–527
Cauchi J, Parikh DH, Samuel M, Gornall P (2006) Does gastroschisis reduction require general anesthesia? A comparative analysis. J Pediatr Surg 41(7):1294–1297
Rao SC, Pirie S, Minutillo C, Gollow I, Dickinson JE, Jacoby P (2009) Ward reduction of gastroschisis in a single stage without general anaesthesia may increase the risk of short-term morbidities: results of a retrospective audit. J Paediatr Child Health 45(6):384–388
Pet GE, Stark RA, Meehan JJ, Javid PJ (2017) Outcomes of bedside sutureless umbilical closure without endotracheal intubation for gastroschisis repair in surgical infants. Am J Surg 213(5):958–962
Briganti V, Luvero D, Gulia C, Piergentili R, Zaami S, Buffone EL et al (2018) A novel approach in the treatment of neonatal gastroschisis: a review of the literature and a single-center experience. J Matern Fetal Neonatal Med 31(9):1234–1240
Bianchi A, Dickson AP, Alizai NK (2002) Elective delayed midgut reduction-No anesthesia for gastroschisis: selection and conversion criteria. J Pediatr Surg 37(9):1334–1336
Stanger J, Mohajerani N, Skarsgard ED (2014) Practice variation in gastroschisis: factors influencing closure technique. J Pediatr Surg 49(5):720–723
Sharp M, Bulsara M, Gollow I et al (2000) Gastroschisis: early enteral feeds may improve outcome. J Paediatr Child Health 36(5):472–476
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data acquisition was performed by MD, AG, and AV. All authors contributed to the analysis and interpretation of data. Drafting of the manuscript was performed by MD, AG, AV, and SJ. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Registration of study and protocol
This review was not registered. Review protocol can be assessed by contacting the corresponding author.'
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] Guidelines
Section and Topic | Item # | Checklist item | Location where item is reported |
|---|---|---|---|
TITLE | |||
Title | 1 | Identify the report as a systematic review | p. 1 |
ABSTRACT | |||
Abstract | 2 | See the PRISMA 2020 for abstracts checklist | p. 3 |
INTRODUCTION | |||
Rationale | 3 | Describe the rationale for the review in the context of existing knowledge | p. 5–6 |
Objectives | 4 | Provide an explicit statement of the objective[s] or question[s] the review addresses | p. 6 |
METHODS | |||
Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses | p. 7 |
Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted | p. 6–7 |
Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used | p. 6–7 |
Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process | p. 8 |
Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process | p. 8 |
Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought [e.g., for all measures, time points, analyses], and if not, the methods used to decide which results to collect | p. 7–8 |
10b | List and define all other variables for which data were sought [e.g., participant and intervention characteristics, funding sources]. Describe any assumptions made about any missing or unclear information | NA | |
Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool[s] used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process | p. 8–9/p. 22 |
Effect measures | 12 | Specify for each outcome the effect measure[s] [e.g., risk ratio, mean difference] used in the synthesis or presentation of results | p. 9–10 |
Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis [e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis [item #5]] | p. 7–8 |
13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions | p. 8–9 | |
13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses | NA | |
13d | Describe any methods used to synthesize results and provide a rationale for the choice[s]. If meta–analysis was performed, describe the model[s], method[s] to identify the presence and extent of statistical heterogeneity, and software package[s] used | p. 9–10 | |
13e | Describe any methods used to explore possible causes of heterogeneity among study results [e.g., subgroup analysis, meta–regression] | NA | |
13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results | NA | |
Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis [arising from reporting biases] | p. 8 |
Certainty assessment | 15 | Describe any methods used to assess certainty [or confidence] in the body of evidence for an outcome | NA |
RESULTS | |||
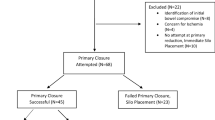
Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram | p. 10 |
16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded | p. 10 | |
Study characteristics | 17 | Cite each included study and present its characteristics | |
Risk of bias in studies | 18 | Present assessments of risk of bias for each included study | p. 10 and 22 |
Results of individual studies | 19 | For all outcomes, present, for each study: [a] summary statistics for each group [where appropriate] and [b] an effect estimate and its precision [e.g., confidence/credible interval], ideally using structured tables or plots | Forrest plot |
Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies | Table 1/p. 22/p. 10 |
20b | Present results of all statistical syntheses conducted. If meta–analysis was done, present for each the summary estimate and its precision [e.g., confidence/credible interval] and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect | NA | |
20c | Present results of all investigations of possible causes of heterogeneity among study results | NA | |
20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results | NA | |
Reporting biases | 21 | Present assessments of risk of bias due to missing results [arising from reporting biases] for each synthesis assessed | NA |
Certainty of evidence | 22 | Present assessments of certainty [or confidence] in the body of evidence for each outcome assessed | Forest plot |
DISCUSSION | |||
Discussion | 23a | Provide a general interpretation of the results in the context of other evidence | p. 13 |
23b | Discuss any limitations of the evidence included in the review | p. 15 | |
23c | Discuss any limitations of the review processes used | p. 15 | |
23d | Discuss implications of the results for practice, policy, and future research | p. 15 | |
OTHER INFORMATION | |||
Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered | p. 16 |
24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared | p. 16 | |
24c | Describe and explain any amendments to information provided at registration or in the protocol | NA | |
Support | 25 | Describe sources of financial or non–financial support for the review, and the role of the funders or sponsors in the review | p. 2 |
Competing interests | 26 | Declare any competing interests of review authors | p. 2 |
Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review | p. 16 |
Appendix 2: MINORS tool for risk-of-bias assessment for descriptive and comparative studies
Vane | Bianchi | Dolgin | Kimble | Duncan | Gore | Kasat | Davies | Cauchi | Rao | Pet | Briganti | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Descriptive | |||||||||||||
Clearly stated aim | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
Inclusion of consecutive patients | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | |
Prospective collection of data | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 1 | |
Endpoints appropriate to aim | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | |
Unbiased assessment of endpoint | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Follow-up period appropriate to aim | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | |
Loss of follow-up < 5% | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
Prospective calculation of study size | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Comparative | |||||||||||||
Adequate control group | NA | NA | NA | NA | NA | NA | NA | 2 | 2 | 2 | 2 | 2 | |
Contemporary group | NA | NA | NA | NA | NA | NA | NA | 1 | 2 | 2 | 2 | 2 | |
Baseline equivalence of groups | NA | NA | NA | NA | NA | NA | NA | 2 | 2 | 1 | 2 | 2 | |
Adequate statistical analyses | NA | NA | NA | NA | NA | NA | NA | 2 | 2 | 2 | 2 | 2 | |
Total | 11 | 9 | 8 | 12 | 8 | 9 | 6 | 19 | 19 | 19 | 18 | 19 | |
Rights and permissions
About this article
Cite this article
Dhane, M., Gervais, AS., Joharifard, S. et al. Avoidance of routine endotracheal intubation and general anesthesia for primary closure of gastroschisis: a systematic review and meta-analysis. Pediatr Surg Int 38, 801–815 (2022). https://doi.org/10.1007/s00383-022-05117-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-022-05117-y