Abstract
Background/purpose
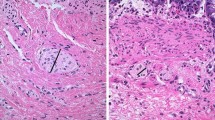
Calretinin immunohistochemistry is now widely used to diagnose Hirschsprung’s disease (HD), since loss of calretinin expression within the mucosa and muscularis mucosae of rectal suction-biopsy is pathognomonic of HD. However, a stippled staining may be observed within hypertrophic nerves in the submucosae in some HD patients. The aim of the study was to test the hypothesis that such findings may announce the beginning of the transitional zone.
Methods
We retrieved 44 consecutive patients (10 girls and 34 boys; median age 6.5 days), diagnosed with aganglionosis on rectal suction biopsies, followed by surgery. According to calretinin immunohistochemistry performed on all paraffin-embedded rectal biopsies, we defined two HD groups: P− showing an absence of any staining within mucosa, muscularis mucosae and submucosa et P+ showing an absence of staining within the mucosa and muscularis mucosae, but a positivity of some submucosal hypertrophic nerves. These data were correlated to the length of total pathological segment (aganglionic and transitional zones) obtained from the original surgery reports.
Results
18/44 patients (40.9 %) belonged to the P+ group and 26/44 (59 %) patients were within the P− group. In the P+ group, the maximal length of the aganglionic zone was 9 cm [mean 4 (1–9)] and the total pathological zone never exceeded 14 cm [mean 8 (3.8–14)]. In the P− group, the maximal length of aganglionic zone was 55.5 cm [mean 11.3 (2.5; 55.5)] and the total pathological zone extended to 59.5 cm [mean 17.75 (4.5; 59.5)]. Aganglionic segment was significantly shorter in the P+ group (p < 0.0001).
Conclusion
Staining of some hypertrophic nerves in the submucosa in suction rectalbiopsy of HD patients using calretinin immunohistochemistry is only encountered in short-segment aganglionosis with a pathological zone always restricted to rectal and sigmoid colon. This information could be crucial for the surgeons in the decision to choose a transanal procedure.



Similar content being viewed by others
References
Guinard-Samuel V, Bonnard A, De Lagausie P et al (2009) Calretinin immunohistochemistry: a simple and efficient tool to diagnose Hirschsprung disease. Mod Pathol 22(10):1379–1384
Barshack I, Fridman E, Goldberg I, Chowers Y, Kopolovic J (2004) The loss of calretinin expression indicates aganglionosis in Hirschsprung’s disease. J Clin Pathol 57(7):712–716
Hobbs CJ, Hanks HGI, Wynne JM (1999) Child abuse and neglect: a clinician’s handbook. Elsevier, Amsterdam
Yin H, Boyd T, Pacheco MC, Schonfeld DJ, Bove KE. Rectal biopsy in children with Down syndrome and chronic constipation: hirschsprung disease vs. non-hirschsprung disease. Pediatr. Dev. Pathol. 2011. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21991983. Accessed 19 April 2012
Holland SK, Ramalingam P, Podolsky RH, Reid-Nicholson MD, Lee JR (2011) Calretinin immunostaining as an adjunct in the diagnosis of Hirschsprung disease. Ann Diagn Pathol 15(5):323–328
Grynspan D, Giassi ACC, Cadonic R et al (2012) Growth-associated protein-43 (GAP-43) expression in ganglionic and aganglionic colon. Pediatr. Dev. Pathol 15(5):428–429. doi:10.2350/12-06-1213-LETR1.1
Kaçar A, Arikök AT, Azili MN, Ekberli Ağirbaş G, Tiryaki T (2012) Calretinin immunohistochemistry in Hirschsprung’s disease: an adjunct to formalin-based diagnosis. Turk J Gastroenterol 23(3):226–233
Melendez E, Goldstein AM, Sagar P, Badizadegan K (2012) Case records of the Massachusetts General Hospital. Case 3-2012. A newborn boy with vomiting, diarrhea, and abdominal distention. N Engl J Med 366(4):361–372
Kannaiyan L et al (2013) Calretinin immunohistochemistry: a new cost-effective and easy method for diagnosis of Hirschsprung’s disease. J Indian Assoc Pediatr Surg 18:66–68
Alexandrescu S, Rosenberg H, Tatevian N (2013) Role of calretinin immunohistochemical stain in evaluation of Hirschsprung disease: an institutional experience. Int J Clin Exp Pathol 6:2955–2961
Kapur RP (2014) Calretinin-immunoreactive mucosal innervation in very short-segment Hirschsprung disease: a potentially misleading observation. Pediatr Dev Pathol 17:28–35
Mahajan JK, Rathod KK, Bawa M, Narasimhan KL (2011) Transanal Swenson’s operation for recto-sigmoid Hirschsprung’s disease. Afr J Paediatr Surg 8(3):301–305
Sookpotarom P, Vejchapipat P (2009) Primary transanal Swenson pull-through operation for Hirschsprung’s disease. Pediatr Surg Int 25(9):767–773
Tannuri ACA, Tannuri U, Romão RLP (2009) Transanal endorectal pull-through in children with Hirschsprung’s disease–technical refinements and comparison of results with the Duhamel procedure. J Pediatr Surg 44(4):767–772
Muller CO, Mignot C, Belarbi N, Berrebi D, Bonnard A (2012) Does the radiographic transition zone correlate with the level of aganglionosis on the specimen in Hirschsprung’s disease? Pediatr Surg Int 28(6):597–601
Proctor ML, Traubici J, Langer JC et al (2003) Correlation between radiographic transition zone and level of aganglionosis in Hirschsprung’s disease: implications for surgical approach. J Pediatr Surg 38(5):775–778
Jamieson DH, Dundas SE, Belushi SA, Cooper M, Blair GK (2004) Does the transition zone reliably delineate aganglionic bowel in Hirschsprung’s disease? Pediatr Radiol 34(10):811–815
Rabah R (2010) Total colonic aganglionosis: case report, practical diagnostic approach and pitfalls. Arch Pathol Lab Med 134:1467–1473
Athow AC, Isabel Filipe M, Drake DP (1990) Problems and advantages of acetylcholinesterase histochemistry of rectal suction biopsies in the diagnosis of Hirschsprung’s disease. J Pediatr Surg 25:520–526
Alizai NK, Batcup G, Dixon MF, Stringer MD (1998) Rectal biopsy for Hirschsprung’s disease: what is the optimum method? Pediatr Surg Int 13(2–3):121–124
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guinard-Samuel, V., Bonnard, A., Peuchmaur, M. et al. A variant pattern of Calretinin immunohistochemistry on rectal suction-biopsies is fully specific of short-segment Hirschsprung’s disease. Pediatr Surg Int 30, 803–808 (2014). https://doi.org/10.1007/s00383-014-3526-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-014-3526-6