Abstract
Introduction
Primary brain tumors are a common cause of morbidity and mortality in children and young people (CYP) globally. Impaired neurocognitive function is a potential severe consequence in primary brain tumor (PBT) survivors. There are no in-depth studies from low- and middle-income countries (LMICs) to inform management and follow-up. The research questions of this study were as follows: Are the sociodemographic factors (lower age of CYP, female gender, low socioeconomic status, low parental education), disease-related factors (high grade of tumor, presence of seizures, presence of hydrocephalous), and treatment-related factors (adjuvant therapy, no surgical intervention, post-treatment seizures, placement of shunts) associated with decline in neurcognition outcomes 12 months post-treatment in CYP with PBTs?
Methods
A prospective cohort study was conducted from November 2020 to July 2023 at the Aga Khan University Hospital and Jinnah Postgraduate Medical Centre, tertiary care hospitals in Karachi, Pakistan. All CYP aged 5 to 21 years with a newly diagnosed PBTs were eligible. The neurocognition assessment was undertaken by a psychologist at two points, i.e., pre-treatment and at 12 months post-treatment using validated tools. The verbal intelligence was assessed by Slosson Intelligence tool, revised 3rd edition (SIT-R3), perceptual reasoning by Raven’s Progressive Matrices (RPM), and the Processing Speed Index by Wechsler Intelligence Scale (WISC V) and Wechsler Adult Intelligence Scale (WAIS-IV). The data were analyzed by STATA version 12 software. Generalized estimating equation (GEE) was used to determine the factors associated with the mean change in 12 months post-treatment verbal and non-verbal neurocognition scores. Unadjusted and adjusted beta coefficients with their 95% confidence intervals were reported.
Results
A total of 48 CYPs with PBTs were enrolled, 23 (48%) of them were lost to follow-up and 10 (21%) died. The remaining 25 (52%) were reassessed 12 months after treatment. On multivariable analysis, a significant decline in verbal intelligence scores at 12 months was predicted by post-treatment seizures beta = − 20.8 (95% CI, − 38.2, − 3.4), mothers having no formal educational status and lower household monthly income. Similarly, a significant decline in perceptual reasoning scores was also predicted by post-treatment seizures beta = − 10.7 (95% CI, − 20.6, − 0.8), mothers having no formal education and having lower household monthly income. Worsening of processing speed scores at 12 months post-treatment were predicted by tumor histology, post-treatment seizures beta = − 33.9 (95% CI, − 47.7, − 20.0), lower educational status of the mother, and having lower household monthly. However, an improvement was seen in processing speed scores after surgical tumor resection.
Conclusion
In this novel study, the post-treatment mean change in verbal and non-verbal neurocognition scores was associated with sociodemographic, tumor, and treatment factors. These findings may have potential implications for targeted early psychological screening of higher risk CYP with PBTs. Identification of these predictors may serve as a foundation for developing more cost-effective treatment thereby alleviating the burden of neurocognitive morbidity. However to establish generalizability, future research should prioritize larger-scale, multicountry studies. (Trial registration: ClinicalTrials.gov Identifier: NCT05709522)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, primary brain tumors (PBTs) are the second most common type of cancer in children and young people (CYP) aged 0–24 years [1]. The World Health Organization (WHO) estimated in 2020 that approximately 10% of all primary brain tumors (PBTs) occur in children and adolescents, ages 0–19 years, and 7% of all PBTs occur in the young adult population aged 20–24 years [2]. Almost 90% of these are from low- and middle-income countries (LMICs) [3]. Anatomically, approximately 60% of the PBTs are located in the posterior fossa the part of the brain under the tentorium and which includes the cerebellum, brainstem, and fourth ventricle, in children 0–15 years [4].
The survival rate of PBTs is increasing due to improvements in detection and treatment. It was reported by the WHO in 2020 that the 5-year relative mortality for all PBTs (0–19 years of age) was 14% [2]. The corresponding figure for young adults (20–24 years of age) was 10% [2]. In 2020, a noteworthy decrease of 14% in mortality rate was observed when compared to 2010 [5]. However, improved survival has been associated with adverse late effects [6,7,8,9].
The term “adverse late effects” encompasses the cumulative negative consequences or unfavorable outcomes that develop over time following the initial treatment. These effects can manifest as a decline in physical, cognitive, emotional, and functional well-being, leading to a reduction in an individual’s overall quality of life (QoL). Often, these negative consequences become apparent months or even years after the initial diagnosis and treatment of tumor [10].
Work in this area suggest that late effects occur in 6 to 21% of childhood cancer survivors aged 0 to 18 years at diagnosis [11, 12]. One of the most devastating consequences is the impairment of neurocognitive functioning from critical thinking, problem-solving, executive control, memory retention, and focused attention. These range from mild to severe, often persisting or even worsening with time (9). It is estimated that 20 to 50% of children aged (6–16 years) with central nervous system (CNS) tumors demonstrate impairment in at least one neurocognitive domain [13]. However, by implementing specialized educational services, precise therapeutic interventions, and customized classroom accommodations, it is possible to significantly alleviate the impact of neurocognitive impairment [10].
A narrative review by Stavinoha et al. showed evidence from a range of studies from high-income countries of the neurocognitive and psychological outcomes in PBT survivors. The studies making up this review, however, were largely cross-sectional or, where longitudinal, without a clearly defined baseline [14]. A study conducted in Canada assessed cognitive outcomes during long-term follow-up among pediatric brain tumors survivors, with repeat testing 1–3 years after initial testing, and estimated a mean decline of a range of cognitive outcomes between 1 and 1.7 standard deviation scores from the baseline pre-treatment values [15]. A meta-analysis of 39 empiric studies reported that these CYP exhibited significant and pervasive impairments in multiple neurocognitive domains and that survivors’ scores on measures of global cognitive ability, verbal and nonverbal, fell nearly 1 standard deviation below normative means [16]. Poon et al. conducted a systematic review including 59 studies assessing the late outcome of childhood cancer in Asian countries [17]. The majority of the relevant studies included survivors of CNS tumors and pediatric acute lymphoblastic leukemia (ALL) [17]. However, only one assessed the neurocognition outcomes in CNS tumor survivors reporting neurocognitive impairment in 10% of the survivors [18].
Importantly, to best of the investigator’s knowledge, there are no in-depth studies evaluating neurocognitive outcome including CYPs with primary brain tumor from low- and middle-income countries (LMICs). Only one study from a tertiary care hospital in Karachi, Pakistan, assessed the neurological, endocrine, hypothalamic complications and survival outcomes in children with craniopharyngioma. However, this study did not assess the neurocognitive outcomes [19]. Due to difference in healthcare infrastructure, socioeconomic factors, cultural differences, and access to advanced diagnostics and treatments in this region, data from high-income countries cannot be extrapolated.
This study included CYP, 5–21 years of age, referred to two tertiary care hospitals in Karachi, Pakistan, and assessed neurocognitive function pre-treatment and reassessed at 12 months post-treatment. The aim of the study was to identify sociodemographic, birth, and disease- and treatment-related factors associated with neurocognitive impairment and deterioration after treatment.
Research questions:
-
1.
Does sociodemographic factors of CYP such as lower age, female gender, and low socioeconomic status cause a decline in the mean neurocognition scores 12 months post-treatment?
-
2.
Does parental sociodemographic factors such as low educational status of parents and low household monthly income cause a decline in the mean neurocognition scores 12 months post-treatment?
-
3.
Are the CYP tumor factors such as high grade of tumor, presence of seizures, and presence of hydrocephalous associated with decline in neurocognition outcomes 12 months post-treatment?
-
4.
Are the CYP treatment factors such as adjuvant therapy, no surgical intervention, post-treatment seizures, and placement of shunts associated with decline in neurcognition outcomes 12 months post-treatment?
Methods
Study design and setting
A prospective cohort study was conducted from November 2020 to July 2023 at the Aga Khan University Hospital (AKUH), a Joint Commission International Accreditation (JCIA‐accredited) private sector tertiary care hospital, in Karachi, Pakistan, and Jinnah Postgraduate Medical Centre (JPMC) a public sector tertiary care hospital, in Karachi, Pakistan.
Study population and eligibility criteria
All CYP, aged 5–21 years, residing in Pakistan with PBTs, presenting at any stage, without previous treatment were eligible. Participants with any known history of psychiatric or neurological illness such as attention deficit hyperactivity disorder (ADHD), autism, and schizophrenia, confirmed by the medical records, or with physical morbidities, were excluded. Children without formal education were also excluded based on the assumption that they might have limited mathematical and language skills, limiting objective evaluation of their cognitive abilities. CYP with complete pre- and post-treatment data were included in the final regression analysis.
Sampling technique
A non-probability purposive sampling technique was employed for selecting the participants. A trained research assistant approached all the CYP with newly diagnosed PBTs presenting to surgical/oncology clinics at AKUH and JPMC during their scheduled appointments. Potential participants were screened for eligibility. If eligible, potential participants and their parents were briefed regarding the scope and nature of the study, as well as the extent of their participation. They were included if patients’ written assent/informed consent and parents’ written consent were provided.
Sample size
The sample size was calculated using standard estimate where n = 8 (CV2)/(PC2) [1 + (1 − PC)2] where PC is the proportionate change in means (PC = (µ0 − µ1)/µ0) and CV is the coefficient of variation (CV = σ0/µ0 = σ1/µ1) [20]. To allow for an inflation rate of 65% (based on previous work) [3], a minimum sample size of 48 CYP with PBTs was estimated to achieve 80% power and to detect at least a 15% change in mean neurocognition scores and 19% or less change in coefficient of variation as reported by Shortman et al., at two sided 5% level of significance [21].
Data collection procedure
Prior to participant’s recruitment, the questionnaire and the neurocognitive tools were pre-tested on 5% of the participants to assess the feasibility. A trained research assistant administered the final questionnaire in Urdu (the national language and official language of Pakistan). Data were gathered on the following variables:
Independent variables
CYP's demographic information including (age, educational status, gender, etc.); parent’s demographic information including (age, educational status, household monthly income); tumor and treatment information that included tumor histopathology and tumor location (supratentorial, infratentorial, suprasellar, and sellar); history of pre-treatment seizures; the presence or absence of hydrocephalus at diagnosis confirmed radiologically by magnetic resonance imaging (MRI); type of treatment (surgical resection, chemotherapy, radiotherapy, and biopsy); and type of surgery , i.e., total resection (100% removal of tumor), maximum safe resection (> 90% removal of tumor while prioritizing safety), and subtotal resection (< 90% removal of tumor), these definitions are based on institutionally established cutoffs, determined through post-MRI assessments of residual tumor. Management by external ventricular drain (EVD) and ventriculoperitoneal shunt (VPS) for those with hydrocephalous at diagnosis.
Outcome variable
Neurocognition
The neurocognition assessment was undertaken by the same psychologist at the pre-treatment and 12 months post-treatment stages. The assessments were undertaken at both the study sites in English or Urdu depending on the understanding and preference of the patient and/or parents.
The verbal intelligence was assessed by Slosson Intelligence tool, revised 3rd edition (SIT-R3). The SIT-R total standard scores were presented as standard scores with a mean (M) of 100 and a standard deviation (SD) of 16. A higher score on the Slosson indicated a more favorable outcome [22]. Although the tool has been translated and adapted for use in Pakistan [23], validation in Urdu is lacking. To address this, a content validity index (CVI) was computed by a panel of experts. These experts assessed the tool for relevance and clarity in relation to the Pakistani cultural context, using a Likert scale of 1 to 4. The CVI scores, which are the average of the content validity ratio (CVR) for each item, were used to determine agreement. The relevance CVI was estimated at 0.90, and the clarity CVI was 0.89. These results reflect a high level of agreement among experts regarding the clarity and relevance of the tool’s content.
Perceptual reasoning was assessed by Raven’s Progressive Matrices (RPM) [24]; a higher score on the RPM indicated a more favorable outcome. It has been previously validated in Pakistan [25].
The Processing Speed Index (PSI) was assessed by Wechsler Intelligence Scale (WISC V) [26, 27] and Wechsler Adult Intelligence Scale (WAIS-IV) [28]. This has been previously validated in Pakistan [29]. A higher score indicated a more favorable outcome.
Ethical considerations
Ethical/institutional review committee approvals from the respective study sites were obtained, AKUH-ERC (ERC#2020–4859-11855) and JPMC (F2-81/2021-GENL/65706/JPMC). Written informed consent was taken directly from patients aged 18 years and older. For those under 18 years of age, both informed assent and parental consent was obtained in English or Urdu as per the understanding of the participants. To ensure confidentiality, interviews were conducted in a separate room. The psychologist provided treatment plan to those identified with a 12 months post-treatment neurocognitive decline at both the study sites.
Statistical analysis
Data were analyzed using STATA version 12 (Stata Texas®). Results were presented as mean and standard deviation (SD)/median and interquartile range (IQR) for quantitative variables. The difference in mean pre-treatment and 12 months post-treatment neurocognitive scores was assessed by paired t-test. Categorical variables were reported as frequency and percentages and were assessed by chi-square or Fisher’s exact test. To determine the association of various independent factors such as child factors (demographic factors), parental factors (educational status, socioeconomic status), and tumor and treatment-related factors with the change in mean neurocognitive scores, generalized estimating equation was applied and the covariates were adjusted. Beta coefficient (unadjusted and adjusted) with 95% confidence interval (CI) was reported. Multicollinearity and plausible interactions were assessed. A p-value of less than 0.2 at univariate analysis and less than 0.05 at multivariable analysis were considered statistically significant.
Results
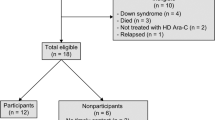
A total of 60 CYP with PBTs were screened for eligibility. We excluded 12 participants. The remaining 48 were enrolled. Among those, 23 (48%) were lost to follow-up, of whom 10 died. At 12 month post-treatment, 25 participants were reviewed. Within this group, one had hearing issues, and six had vision issues. Consequently, the follow-up data on verbal intelligence was obtained from 24 participants, data on perceptual reasoning from 19 participants, and data on processing speed from 19 participants (Fig. 1).
Sociodemographic and birth-related factors
The mean age of the recruited CYP was 12.8 (4.6) years. Sixty percent were males. The median household monthly income was 106 USD (Table 1).
Tumor and treatment-related factors
There was no significant difference in CYP's mean age at diagnosis 12.5 (4.5) years and at presentation to the hospital for treatment 12.8 (4.6) years. Twenty two out of forty eight (46%) required a ventriculoperitoneal shunt (VPS) or external ventricular drain (EVD). Thirty eight out of 48 (79%) underwent surgery (Table 2).
Post-treatment seizures were reported in 4 out of 25 (16%) CYP. A higher percentage with low-grade tumors (33%) versus high-grade tumors (7%) underwent total resection. After treatment, 14 out 25 (56%) discontinued their education, and 11 out 14 (79%) who discontinued were those who belonged to households with monthly income of less than USD 159. Among the 10/48 (21%) patients who expired, 5 out of 10 (50%) had medulloblastoma, 1 out 10 (10%) had pituitary adenoma, and 1 out of 10 (10%) had craniopharyngioma (Table 2).
Pre-treatment neurocognition scores
The pre-treatment mean verbal neurocognitive score (81 ± 19.5) was 1 standard deviation (SD) below the normative mean, while the pre-treatment mean perceptual reasoning score (94 ± 15.3) was 0.41 SD below the normative mean and the mean processing speed (63 ± 14.4) was 2 SD below the normative mean. There was statistically significant positive correlation between verbal intelligence score and perceptual reasoning score (r = 0.5; p = ≤ 0.002). Similarly, a significant positive correlation was also observed between verbal intelligence score and processing speed score (r = 0.5; p = 0.001). Furthermore, a significant positive correlation was observed between perceptual reasoning score and process speed score (r = 0.4; p-value = 0.047). The verbal intelligence score of 70% of the participants was below average, while perceptual reasoning score of 44% of the participants was below average (Supplementary 1).
Post-treatment neurocognition scores
The post-treatment mean verbal intelligence score (84 ± 22.9) was one standard deviation below the normative mean. The post-treatment mean perceptual reasoning score (92 ± 14.7) was 0.61 standard deviation below the normative mean, and the post-treatment mean processing speed score (70 ± 15.5) was 2 SD below the normative mean. No statistically significant difference was observed in the mean change in neurocognition scores at 12 months post-treatment reassessment among the participants.
Univariable analysis
On univariate analysis, those with post-treatment seizures had a statistically significant decline in verbal intelligence, perceptual reasoning, and processing speed scores (p-values < 0.2). Furthermore, a statistically significant increase in verbal intelligence scores and processing speed scores was observed in those who underwent surgical resection (p-value = 0.1 and p-value = 0.01), respectively. Individuals whose parents possessed higher educational qualifications and those who belonged to households with higher monthly income had a significant improvement in verbal intelligence, perceptual reasoning, and processing speed scores (p-values < 0.001). A significant decline was observed in verbal intelligence and processing speed scores in patients with tumor histopathology of ependymoma, craniopharyngioma, glioblastoma, and medulloblastoma (p-values ≤ 0.2). Finally, those with hydrocephalous had a significant decline in processing speed scores (p-value = 0.1) (Table 3).
Multivariable analysis
On multivariable analysis, a statistically significant decline in verbal intelligence at 12 months was predicted by post-treatment seizures beta = − 20.8 (95% CI, − 38.2, − 3.4), mothers having no formal education beta = − 18.2 (95% CI, − 33.7, − 2.8) and having lower household monthly income of < USD 53 beta = − 29.7 (95% CI, − 47.9, − 11.6) and USD 159–320 beta = − 21.1 (95% CI, − 39.7, − 2.5) as compared to those with income of > USD 320 (Table 4, Supplementary 2).
Similarly, a statistically significant decline in perceptual reasoning scores was predicted by post-treatment seizures beta = − 10.7 (95% CI, − 20.6, − 0.8), mothers having no formal education beta = − 9.8 (− 18.6, − 1.0) and having lower household monthly income of < USD 53 beta = − 22 (95% CI, 33.5, − 10.5), USD 53–159 beta = − 11.9 (95% CI, − 20.4, − 3.4), and USD 159–320 beta = − 11 (95% CI, − 20.6, − 1.3) as compared to those with income of > USD 320 (Table 4, Supplementary 2).
Furthermore, the processing speed scores were significantly decreased at 12 months among participants with the following tumor histopathology: ependymoma, glioblastoma, and craniopharyngioma when compared to pilocytic astrocytoma. Moreover, a statistically significant decline in processing speed scores were also predicted by post-treatment seizures beta = − 33.9 (95% CI, − 47.7, − 20.0), mothers having no education beta = − 7.0 (− 14.8, 0.7), primary education beta = − 14.6 (− 25.8, − 3.4), and secondary education beta = − 11.2 (− 19.7, − 2.8) compared to those with educational status of higher secondary and above. A significant decline was also observed in those having household monthly income of < USD 53 beta = − 30.5 (95% CI, − 42.3, − 18.7), USD 53–159 beta = − 5.8 (95% CI, − 11.5, − 0.1), and USD 159–320 beta = − 10.5 (95% CI, − 18.8, − 2.1) compared to those with an income of > 320 USD. Conversely, the processing speed scores were significantly increased among those who underwent surgical tumor resection, i.e., total resection beta = − 30.5 (95% CI, − 42.3, − 18.7), subtotal resection beta = − 5.8 (95% CI, − 11.5, − 0.1), and maximum safe resection beta = − 10.5 (95% CI, − 18.8, − 2.1) compared to those who underwent no surgical resection (Table 4, Supplementary 2).
Discussion
The studies conducted in high-income countries as reported in a narrative review by Stavinoha et al. are largely cross-sectional or, where longitudinal, without a clearly defined baseline [14]. However, from the Asian context, a systematic review of 59 studies by Poon et al. [17] reported long-term outcomes of lymphoblastic leukemia and CNS tumors survivors, and only one study assessed the neurocognition outcomes reporting impairment in 10% of CNS tumor survivors [18]. From Pakistan, only one study assessed the neurological, endocrine, hypothalamic complications and survival outcomes in children with craniopharyngioma. However, this study did not assess the neurocognitive outcomes [19]. Therefore, this prospective cohort study aimed to identify the factors associated with neurocognitive impairment among children and young people (CYP) with primary brain tumor after treatment in Pakistan.
The study found an association of tumor type with neurocognition outcomes in CYP with PBTs. There was a significant decline in processing speed scores in patients with glioblastoma (grade 4) and ependymoma (grades 2 and 3) compared to pilocytic astrocytoma (grade 1). These findings align with those reported by Klein et al. who found a higher prevalence of cognitive impairment in malignant brain tumors (grades 3 and 4) compared to low-grade tumors, independent of tumor volume, patient’s age, and tumor location [30]. The infiltrative and invasive nature of malignant tumors may impede neural plasticity by releasing tumor humoral substances that impairs cortical function, potentially causing decline in processing speed of the brain [31].
A statistically significant decline in processing speed was also observed in those with craniopharyngiomas compared to those with pilocytic astrocytomas. This is consistent with the findings by Merchant et al. who observed attention-related challenges in this group [32]. Craniopharyngiomas are histologically benign but, given their anatomical position in the sella turcica, frequently involve compression of adjacent critical structures such as the hypothalamus, pituitary glands, cranial nerves, and circle of Willis [33]. These tumors usually cause prominent emotional, cognitive, and behavioral disturbances derived from hypothalamic dysfunction [34]. Its proximity to the pituitary may also lead to endocrine irregularities [35]. Endocrinopathy plays a significant role in cognitive outcomes in craniopharyngioma too [36]. Although the study did not assess the endocrine profile of the patients, but a study from Pakistan reported that 37 (75%) patients with craniopharyngioma were found to have panhypopituitarism and required long-term hormonal replacement therapy 1 year after surgery [19].
This study also found a significant improvement in processing speed scores of those who underwent surgical resection as compared to those who did not undergo surgical resection. The current literature suggests that surgical intervention can mitigate various issues arising from tumor including relief of mass-related pressure and a reduction of swelling that leads to faster restoration of normal brain function and improvement in cerebrospinal fluid flow [37, 38]. This is consistent with Hendrix et al., who reported a low risk of cognitive deterioration following surgical resection. The neurocognitive function appeared to recover within the initial 2 months after the surgery in their sample [38]. Teixidor et al. also reported long-term improvement of verbal memory, after a transient immediate postoperative worsening, following surgical resection [39].
In this study, CYP with post-treatment seizures had a statistically significant decline in verbal and non-verbal neurocognitive scores. Seizures may have an impact on brain maturation and cognitive function in the CYP, the most vulnerable period of brain development [40, 41]. The study findings were consistent with a study by Phillips et al. which suggested that resolution of seizures among patients with CNS tumors lead to an improvement in neurocognition outcomes [42].
There was statistically significant decline in verbal and non-verbal neurocognitive domains among CYP with lower socioeconomic status, particularly in cases where the patients’ mothers had low or no formal education. These findings are consistent with Ellenberg et al., Ward et al., and Carlson-Green et al. all of which emphasized that environmental factors, including socioeconomic status (SES), loss of schooling and socialization, and alterations in family psychosocial functioning, can have an impact neurocognitive outcomes [43, 44]. A study by Nathan et al. also showed that parental education is a predictor of neurocognition outcomes [45]. It is possible that many children from higher (SES) families benefit from greater parental support, more cognitively stimulating home environments, and increased ability of parents to advocate for the unique needs of their children. Furthermore, it is reasonable to assume that more affluent educational institutions possess better resources to offer specialized assistance to children struggling with treatment-induced neurocognitive impairments. This suggests a role for educational infrastructure in addressing such challenges and underscores the need for equitable access to these resources across all segments of society [46].
Study strengths
There were several strengths of the study: Firstly, the longitudinal study design with baseline information of the patients helped examining the neurocognition changes over time within the same group of patients. Secondly, study employed validated neurocognition assessment tools, ensuring the reliability and accuracy of the data collected. Thirdly, the psychologist devised a treatment plan for those CYP who were identified having a significant decline in 12 months post-treatment neurocognition scores. Lastly, given the similarity between those who were lost to follow-up at the 12 months post-treatment with those who were reassessed at that point, therefore attrition bias is unlikely to explain the findings (Supplementary 3).
Study limitations
The study had a number of limitations. Firstly, the relatively small sample size restricted its statistical power. Secondly, the CYP population displayed heterogeneity with respect to tumor histopathology. Thirdly, the assessment was performed at only two time points, pre-treatment and at 12 months post-treatment: multiple assessments could have yielded more informative results. Furthermore, we did not evaluate the endocrine profile of the patients, a crucial aspect of adverse late effects and disturbances of which could mediate outcome. Fourthly, there was a higher proportion of females among those who were lost to follow-up compared to males. Lastly, we cannot exclude a degree of selection bias as those with operable conditions may naturally have more favorable long-term outcomes.
Conclusions
In this novel study, the post-treatment mean change in verbal and non-verbal neurocognition scores was associated with sociodemographic, tumor, and treatment factors. These findings may have potential implications for targeted early psychological screening of higher risk CYP with PBTs. Identification of these predictors may serve as a foundation for developing more cost-effective treatment thereby alleviating the burden of neurocognitive morbidity. However, to establish generalizability, future research should prioritize larger-scale, multicountry studies.
Study implications
A crucial aspect highlighted by the study is identifying factors associated with neurocognitive outcomes in the post-operative phase that would allow timely interventions in CYP with PBTs. This early response has the potential to prevent or mitigate cognitive decline, ultimately leading to better long-term outcomes for CYP affected by PBTs. By understanding the specific neurocognitive outcomes, clinicians can tailor interventions to address cognitive deficits, thus facilitating the development of personalized treatment plans that would enhance overall well-being. However, to achieve these goals, a sustained collaboration is imperative among various stakeholders, including psychologists, neuropsychologists, caregivers, rehabilitation facilities, and schools. This collaborative effort spans from the initial diagnosis to long-term follow-ups, ensuring a comprehensive and cohesive approach to the well-being of these children.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CYP:
-
Children and young people
- PBT:
-
Primary brain tumor
- WHO:
-
World Health Organization
- QoL:
-
Quality of life
- CNS:
-
Central nervous system
- AKUH:
-
Aga Khan University Hospital
- JCIA:
-
Joint Commission International Accreditation
- JPMC:
-
Jinnah Postgraduate Medical Centre
- ADHD:
-
Attention deficit hyperactivity disorder
- PC:
-
Proportionate change in means
- CV:
-
Coefficient of variation
- SITS-R3:
-
Slosson Intelligence tool, revised 3rd edition
- M:
-
Mean
- SD:
-
Standard deviation
- CVI:
-
Content validity index
- CVR:
-
Content validity ratio
- RPM:
-
Raven’s Progressive Matrices
- PSI:
-
Processing Speed Index
- WISC:
-
Wechsler Intelligence Scale
- WAIS:
-
Wechsler Adult Intelligence Scale
- ERC:
-
Ethics review committee
- IQR:
-
Interquartile range
- CI:
-
Confidence interval
- VPS:
-
Ventriculoperitoneal shunt
- r :
-
Correlation coefficient
References
Sung H, Ferlay J, Siegel RL, et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2020). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today/
Riaz Q et al (2019) Intracranial tumors in children: a 10-year review from a single tertiary health-care center. Child’s Nervous System 35(12):2347–2353
Dörner L et al (2007) Posterior fossa tumors in children: how long does it take to establish the diagnosis? Childs Nerv Syst 23(8):887–890
Hossain MJ et al (2021) Epidemiology and prognostic factors of pediatric brain tumor survival in the US: evidence from four decades of population data. Cancer Epidemiol 72:101942
King AA et al (2017) Long-term neurologic health and psychosocial function of adult survivors of childhood medulloblastoma/PNET: a report from the Childhood Cancer Survivor Study. Neuro Oncol 19(5):689–698
Schulte F et al (2018) Social adjustment in adolescent survivors of pediatric central nervous system tumors: a report from the C hildhood C ancer S urvivor S tudy. Cancer 124(17):3596–3608
Willard VW et al (2019) Trajectories of psychosocial and cognitive functioning in pediatric patients with brain tumors treated with radiation therapy. Neuro Oncol 21(5):678–685
Armstrong GT et al (2009) Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst 101(13):946–958
Rey-Casserly C, Diver T (2019) Late effects of pediatric brain tumors. Curr Opin Pediatr 31(6):789–796
Geenen MM et al (2007) Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297(24):2705–2715
Oeffinger KC et al (2006) Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355(15):1572–1582
Margelisch K et al (2015) Cognitive dysfunction in children with brain tumors at diagnosis. Pediatr Blood Cancer 62(10):1805–1812
Stavinoha PL et al (2018) Neurocognitive and psychosocial outcomes in pediatric brain tumor survivors. Bioengineering 5(3):73
Brière ME et al (2008) Cognitive outcome in pediatric brain tumor survivors: delayed attention deficit at long-term follow-up. Pediatr Blood Cancer 50(2):337–340
Robinson KE et al (2010) A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer 55(3):525–531
Poon LHJ et al (2019) Clinical ascertainment of health outcomes in Asian survivors of childhood cancer: a systematic review. J Cancer Surviv 13:374–396
Liang S-Y et al (2013) Neuropsychological functions and quality of life in survived patients with intracranial germ cell tumors after treatment. Neuro Oncol 15(11):1543–1551
Memon, F., Humayun, K.N., Riaz, Q. et al. (2023) Pediatric craniopharyngioma: a 20-year study on epidemiological features, clinical presentation, and survival outcomes in a tertiary care center from LMIC. Childs Nerv Syst. https://doi.org/10.1007/s00381-023-06177-8
Van Belle G (2011) Statistical rules of thumb. Vol. 699. John Wiley & Sons.
Shortman RI et al (2014) Cognitive function in children with brain tumors in the first year after diagnosis compared to healthy matched controls. Pediatr Blood Cancer 61(3):464–472
Slosson RL, Nicholson C, Hibpshman T (1991) Slosson intelligence test, revised (SIT-R3). Slosson Education Publications, Austin, TX
Jaffar R, Ali AZ (2021) Using urdu-english code-switching in the the translation of slosson intelligence test. Pak J Educ Res 4(4). https://doi.org/10.52337/pjer.v4i4.367
Raven J (2008) General introduction and overview: The Raven Progressive Matrices Tests: Their theoretical basis and measurement model. In: Raven, J. & Raven, J. (eds.) Uses and Abuses of Intelligence: Studies Advancing Spearman and Raven’s Quest for Non-Arbitrary Metrics. Unionville, New York: Royal Fireworks Press; Edinburgh, Scotland: Competency Motivation Project; Budapest, Hungary: EDGE 2000; Cluj Napoca, Romania: Romanian Psychological Testing Services SRL (Chapter 1, pp. 17–68)
Domino G, Domino ML (2006) Psychological testing: an introduction. Cambridge University Press. https://books.google.com.pk/bookid=OiKau0aqtsYC&lpg=PT553&ots=w0Un5g1-aK&lr&pg=PT553#v=onepage&q&f=false
Na SD, Burns TG (2016) Wechsler intelligence scale for children-V: Test review. Applied Neuropsychology: Child 5(2):156–160
Manual for the Wechsler Intelligence Scale for Children (3rd ed.) (1991) Psychological Corporation, San Antonio, TX
Scale WAI (1955) Wechsler Adult Intelligence Scale. San Antonio, TX: Harcourt Assessment, Inc. Wehman P, Kreutzer J, Sale P, West M, Morton M, Diambra J (1989) Cognitive impairment and remediation: Implications for employment following traumatic brain injury. J Head Trauma Rehabil 4(3):6675
Ambreen SAIMA, Ajmal M (2014) Wechsler Intelligence Scale for Children-(WISC-IV): adaptation, translation, and standardization in Pakistan (Doctoral dissertation, Doctoral Thesis, National Institute of Psychology, Quaid-e-Azam University, Islamabad] retrieved from: http://prr.hec.gov.pk/jspui/bitstream/123456789/7136/1/Saima_Ambreen_Psychology_20015_QAU_ISD.pdf
Klein M (2016) Lesion momentum as explanation for preoperative neurocognitive function in patients with malignant glioma. Neuro-oncology 18(12):1595–1596
Gempt J et al (2017) Factors influencing neurocognitive function in patients with neuroepithelial tumors. Sci Rep 7(1):17764
Merchant TE et al (2011) Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol 29(36):4776
Zada G et al (2013) Prevalence of neurobehavioral, social, and emotional dysfunction in patients treated for childhood craniopharyngioma: a systematic literature review. PLoS ONE 8(11):e76562
Pascual JM et al (2018) Craniopharyngiomas primarily involving the hypothalamus: a model of neurosurgical lesions to elucidate the neurobiological basis of psychiatric disorders. World Neurosurgery 120:e1245–e1278
Hamblin R, Tsermoulas G, Karavitaki N (2021) Craniopharyngiomas. La Presse Médicale 50(4):104078
Di Pinto M et al (2012) Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys 84(3):e363–e369
Tucha O et al (2003) Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. J Neurosurg 98(1):21–31
Hendrix P et al (2017) Neurocognitive function surrounding the resection of frontal WHO grade I meningiomas: a prospective matched-control study. World neurosurgery 98:203–210
Teixidor P et al (2007) Assessment of verbal working memory before and after surgery for low-grade glioma. J Neurooncol 81:305–313
Badawy R et al (2009) The peri-ictal state: cortical excitability changes within 24 h of a seizure. Brain 132(4):1013–1021
Lin H et al (2009) Recurrent seizures induce a reversible impairment in a spatial hidden goal task. Hippocampus 19(9):817–827
Phillips NS et al (2022) Seizures’ impact on cognition and quality of life in childhood cancer survivors. Cancer 128(1):180–191
Ellenberg L et al (2009) Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology 23(6):705
Ward C et al (2009) Treatment factors associated with outcomes in children less than 3 years of age with CNS tumours. Child’s Nervous System 25:663–668
Nathan PC et al (2007) Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med 161(8):798–806
Ullrich NJ, Embry L (2012). Neurocognitive dysfunction in survivors of childhood brain tumors. In Seminars in pediatric neurology 19(1):35–42. WB Saunders
Funding
Open access funding provided by Uppsala University. The study was funded by the Faculty Development Award (FDA), Aga Khan University Karachi, Pakistan. The funders have no role in the preparation of data or the manuscript.
Author information
Authors and Affiliations
Contributions
NZ conceived the study, analyzed data, and critically reviewed the manuscript. NB and TM overlooked the study and critically reviewed the manuscript. IA critically reviewed the “Result” section of the manuscript. SAE and NM were the subject experts. FA and AH collected the data. FJ and LR were the collaborators of the study. MM was the psychologist and overlooked the psychological assessment results. MNM, SA, and SK critically reviewed the manuscript. All authors have contributed intellectually to this manuscript and have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zahid, N., Enam, S.A., Mårtensson, T. et al. Predictors of neurocognition outcomes in children and young people with primary brain tumor presenting to tertiary care hospitals of Karachi, Pakistan: a prospective cohort study. Childs Nerv Syst 40, 1707–1719 (2024). https://doi.org/10.1007/s00381-024-06306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-024-06306-x