Abstract
Introduction
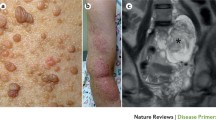
Neurofibromatosis type 1 (NF1) is a frequent autosomal dominant disorder characterised by café-au-lait maculae (CALM), skinfold freckling, iris Lisch nodules and benign peripheral nerve sheath tumours (neurofibromas).
Mechanism
The NF1 gene is a tumour suppressor gene and NF1 individuals have an increased risk for a long list of tumours, all resulting from a second hit in the normal copy of the NF1 gene. Remarkably, some non-tumour phenotypes such as CALM and pseudarthrosis are also caused by a “second hit”. Germline mutations inactivating the NF1 gene show a large variability in genetic mechanisms ranging from single-nucleotide substitutions and somatic mosaicism to large deletions affecting neighbouring genes. Molecular confirmation of the clinical diagnosis is becoming increasingly more important to differentiate NF1 from other syndromes such as Legius syndrome, to investigate genotype-phenotype correlations relevant in 10% of cases and to detect somatic mosaicism.
Surveillance and therapy
Some degree of learning difficulties, attention deficit and social problems are observed in most children and affect quality of life. There is a large individual variability in complications and the evolution of the disease is difficult to predict. Specialised outpatient clinics for children have been widely established and are important for surveillance and guidance. Regular surveillance is also important for adolescents and adults because many tumour complications can be detected by whole-body MRI and treated even before symptoms develop and irreversible damage occurs. Recent data on nodular plexiform neurofibromas with continued growth in adolescents and young adults show that many of these tumours are premalignant lesions called atypical neurofibromatous neoplasm of uncertain biological potential (ANNUBP). Specific surveillance and timely local resection of these benign peripheral nerve sheath tumours might be important to prevent malignant degeneration. In the last years, targeted therapy with MEK inhibitors has shown promise to treat unresectable and symptomatic plexiform neurofibromas. Many more challenges remain to find the best way to monitor children and adults for potential complications and to find a satisfying cure for many complications in this disorder.


Similar content being viewed by others
References
Uusitalo E, Leppävirta J, Koffert A, Suominen S, Vahtera J, Vahlberg T, Pöyhönen M, Peltonen J, Peltonen S (2015) Incidence and mortality of neurofibromatosis: a total population study in Finland. J Invest Dermatol 135:904–906
Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F (2010) Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A 152A:327–332
Kallionpää RA, Uusitalo E, Leppävirta J, Pöyhönen M, Peltonen S, Peltonen J (2018) Prevalence of neurofibromatosis type 1 in the Finnish population. Genet Med 20:1082–1086
Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ (2017) Neurofibromatosis type 1. Nat Rev Dis Primers 3:17004
(1988) National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, Md., USA, July 13–15, 1987. Neurofibromatosis 1: 172–178
Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL (1990) Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science 249:181–186
Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK, Culver M, Carey JC, Copeland NG, Jenkins NA (1990) Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell 62:187–192
LEGIUS E, MARCHUK D, COLLINS F, GLOVER T (1993) Somatic deletion of the neurofibromatosis type-1 gene in a neurofibrosarcoma supports a tumor suppressor gene hypothesis. Nat Genet 3:122–126
Brems H, Beert E, de Ravel T, Legius E (2009) Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol 10:508–515
Messiaen LM, Callens T, Mortier G, Beysen D, Vandenbroucke I, Van Roy N, Speleman F, Paepe AD (2000) Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat 15:541–555
Vogt J, Bengesser K, Claes KB, Wimmer K, Mautner VF, van Minkelen R, Legius E, Brems H, Upadhyaya M, Högel J, Lazaro C, Rosenbaum T, Bammert S, Messiaen L, Cooper DN, Kehrer-Sawatzki H (2014) SVA retrotransposon insertion-associated deletion represents a novel mutational mechanism underlying large genomic copy number changes with non-recurrent breakpoints. Genome Biol 15:R80
Wimmer K, Yao S, Claes K, Kehrer-Sawatzki H, Tinschert S, De Raedt T, Legius E, Callens T, Beiglböck H, Maertens O, Messiaen L (2006) Spectrum of single- and multiexon NF1 copy number changes in a cohort of 1,100 unselected NF1 patients. Genes Chromosomes Cancer 45:265–276
Wimmer K, Callens T, Wernstedt A, Messiaen L (2011) The NF1 gene contains hotspots for L1 endonuclease-dependent de novo insertion. PLoS Genet 7:e1002371
Kehrer-Sawatzki H, Mautner VF, Cooper DN (2017) Emerging genotype-phenotype relationships in patients with large NF1 deletions. Hum Genet 136:349–376
Maertens O, De Schepper S, Vandesompele J, Brerns H, Heyns I, Janssens S, Speleman F, Legius E, Messiaen L (2007) Molecular dissection of isolated disease features in mosaic neurofibromatosis type 1. Am J Hum Genet 81:243–251
De Schepper S, Maertens O, Callens T, Naeyaert JM, Lambert J, Messiaen L (2008) Somatic mutation analysis in NF1 café au lait spots reveals two NF1 hits in the melanocytes. J Invest Dermatol 128:1050–1053
Brekelmans C, Hollants S, De Groote C, Sohier N, Maréchal M, Geris L, Luyten FP, Ginckels L, Sciot R, de Ravel T, De Smet L, Lammens J, Legius E, Brems H (2019) Neurofibromatosis type 1-related pseudarthrosis: beyond the pseudarthrosis site. Hum Mutat 40:1760–1767
Sant DW, Margraf RL, Stevenson DA, Grossmann AH, Viskochil DH, Hanson H, Everitt MD, Rios JJ, Elefteriou F, Hennessey T, Mao R (2015) Evaluation of somatic mutations in tibial pseudarthrosis samples in neurofibromatosis type 1. J Med Genet 52:256–261
Stevenson DA, Zhou H, Ashrafi S, Messiaen LM, Carey JC, D’Astous JL, Santora SD, Viskochil DH (2006) Double inactivation of NF1 in tibial pseudarthrosis. Am J Hum Genet 79:143–148
Bollag G, Clapp DW, Shih S, Adler F, Zhang YY, Thompson P, Lange BJ, Freedman MH, McCormick F, Jacks T, Shannon K (1996) Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet 12:144–148
Simanshu DK, Nissley DV, McCormick F (2017) RAS proteins and their regulators in human disease. Cell 170:17–33
Maertens O, Cichowski K (2014) An expanding role for RAS GTPase activating proteins (RAS GAPs) in cancer. Adv Biol Regul 55:1–14
Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein PE, MacCollin M, Cichowski K (2006) A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell 10:459–472
Akshintala S, Baldwin A, Liewehr DJ, Goodwin A, Blakeley JO, Gross AM, Steinberg SM, Dombi E, Widemann BC (2020) Longitudinal evaluation of peripheral nerve sheath tumors in neurofibromatosis type 1: growth analysis of plexiform neurofibromas and distinct nodular lesions. Neuro-Oncology
Beert E, Brems H, Daniels B, De Wever I, Van Calenbergh F, Schoenaers J, Debiec-Rychter M, Gevaert O, De Raedt T, Van den Bruel A, de Ravel T, Cichowski K, Kluwe L, Mautner V, Sciot R, Legius E (2011) Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes & Cancer 50:1021–1032
Miettinen MM, Antonescu CR, Fletcher CDM, Kim A, Lazar AJ, Quezado MM, Reilly KM, Stemmer-Rachamimov A, Stewart DR, Viskochil D, Widemann B, Perry A (2017) Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol 67:1–10
Higham CS, Dombi E, Rogiers A, Bhaumik S, Pans S, Connor SEJ, Miettinen M, Sciot R, Tirabosco R, Brems H, Baldwin A, Legius E, Widemann BC, Ferner RE (2018) The characteristics of 76 atypical neurofibromas as precursors to neurofibromatosis 1 associated malignant peripheral nerve sheath tumors. Neuro-Oncology 20:818–825
Nelson CN, Dombi E, Rosenblum JS, Miettinen MM, Lehky TJ, Whitcomb PO, Hayes C, Scott G, Benzo S, Widemann BC, Chittiboina P (2019) Safe marginal resection of atypical neurofibromas in neurofibromatosis type 1. J Neurosurg:1–11
Bernthal NM, Putnam A, Jones KB, Viskochil D, Randall RL (2014) The effect of surgical margins on outcomes for low grade MPNSTs and atypical neurofibroma. J Surg Oncol 110:813–816
Evans DGR, Salvador H, Chang VY, Erez A, Voss SD, Schneider KW, Scott HS, Plon SE, Tabori U (2017) Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Cancer Res 23:e46–e53
Ferner RE, Golding JF, Smith M, Calonje E, Jan W, Sanjayanathan V, O’Doherty M (2008) [18F]2-Fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol 19:390–394
Warbey VS, Ferner RE, Dunn JT, Calonje E, O’Doherty MJ (2009) [18F]FDG PET/CT in the diagnosis of malignant peripheral nerve sheath tumours in neurofibromatosis type-1. Eur J Nucl Med Mol Imaging 36:751–757
Pemov A, Hansen NF, Sindiri S, Patidar R, Higham CS, Dombi E, Miettinen MM, Fetsch P, Brems H, Chandrasekharappa S, Jones K, Zhu B, Wei JS, Mullikin JC, Wallace MR, Khan J, Legius E, Widemann BC, Stewart DR, Program NCS, Laboratory NDCGR (2019) Low mutation burden and frequent loss of CDKN2A/B and SMARCA2, but not PRC2, define pre-malignant neurofibromatosis type 1-associated atypical neurofibromas. Neuro-Oncology 21:981–992
Legius E, Dierick H, Wu R, Hall B, Marynen P, Cassiman J, Glover T (1994) TP53 mutations are frequent in malignant NFI tumors. Genes Chromosomes Cancer 10:250–255
De Raedt T, Beert E, Pasmant E, Luscan A, Brems H, Ortonne N, Helin K, Hornick JL, Mautner V, Kehrer-Sawatzki H, Clapp W, Bradner J, Vidaud M, Upadhyaya M, Legius E, Cichowski K (2014) PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 514:247–251
Zhang M, Wang Y, Jones S, Sausen M, McMahon K, Sharma R, Wang Q, Belzberg AJ, Chaichana K, Gallia GL, Gokaslan ZL, Riggins GJ, Wolinksy JP, Wood LD, Montgomery EA, Hruban RH, Kinzler KW, Papadopoulos N, Vogelstein B, Bettegowda C (2014) Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet 46:1170–1172
Lee W, Teckie S, Wiesner T, Ran L, Prieto Granada CN, Lin M, Zhu S, Cao Z, Liang Y, Sboner A, Tap WD, Fletcher JA, Huberman KH, Qin LX, Viale A, Singer S, Zheng D, Berger MF, Chen Y, Antonescu CR, Chi P (2014) PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet 46:1227–1232
De Raedt T, Brems H, Wolkenstein P, Vidaud D, Pilotti S, Perrone F, Mautner V, Frahm S, Sciot R, Legius E (2003) Elevated risk for MPNST in NF1 microdeletion patients. Am J Hum Genet 72:1288–1292
Koczkowska M, Callens T, Chen Y, Gomes A, Hicks AD, Sharp A, Johns E, Uhas KA, Armstrong L, Bosanko KA, Babovic-Vuksanovic D, Baker L, Basel DG, Bengala M, Bennett JT, Chambers C, Clarkson LK, Clementi M, Cortés FM, Cunningham M, D’Agostino MD, Delatycki MB, Digilio MC, Dosa L, Esposito S, Fox S, Freckmann ML, Fauth C, Giugliano T, Giustini S, Goetsch A, Goldberg Y, Greenwood RS, Griffis C, Gripp KW, Gupta P, Haan E, Hachen RK, Haygarth TL, Hernández-Chico C, Hodge K, Hopkin RJ, Hudgins L, Janssens S, Keller K, Kelly-Mancuso G, Kochhar A, Korf BR, Lewis AM, Liebelt J, Lichty A, Listernick RH, Lyons MJ, Maystadt I, Martinez Ojeda M, Mc Dougall C, McGregor LK, Melis D, Mendelsohn N, Nowaczyk MJM, Ortenberg J, Panzer K, Pappas JG, Pierpont ME, Piluso G, Pinna V, Pivnick EK, Pond DA, Powell CM, Rogers C, Ruhrman Shahar N, Rutledge SL, Saletti V, Sandaradura SA, Santoro C, Schatz UA, Schreiber A, Scott DA, Sellars EA, Sheffer R, Siqveland E, Slopis JM, Smith R, Spalice A, Stockton DW, Streff H, Theos A, Tomlinson GE, Tran G, Trapane PL, Trevisson E, Ullrich NJ, Van den Ende J, Schrier Vergano SA, Wallace SE, Wangler MF, Weaver DD, Yohay KH, Zackai E, Zonana J, Zurcher V, KBM C, Eoli M, Martin Y, Wimmer K, De Luca A, Legius E, Messiaen LM (2020) Clinical spectrum of individuals with pathogenic NF1 missense variants affecting p.Met1149, p.Arg1276, and p.Lys1423: genotype-phenotype study in neurofibromatosis type 1. Hum Mutat 41:299–315
Lopez-Correa C, Brems H, Lazaro C, Estivill X, Clementi M, Mason S, Marynen P, Legius E (1999)Inter-chromosomal homologous recombination could mediate large NF1 gene deletions. Am J Hum Genet 65:A8–A8
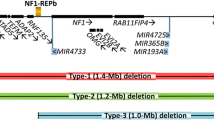
De Raedt T, Stephens M, Heyns I, Brems H, Thijs D, Messiaen L, Stephens K, Lazaro C, Wimmer K, Kehrer-Sawatzki H, Vidaud D, Kluwe L, Marynen P, Legius E (2006) Conservation of hotspots for recombination in low-copy repeats associated with the NF1 microdeletion. Nat Genet 38:1419–1423
Descheemaeker M, Roelandts K, De Raedt T, Brems H, Fryns J, Legius E (2004) Intelligence in individuals with a neurofibromatosis type 1 microdeletion. Am J Med Genet A 131A:325–326
Spiegel M, Oexle K, Horn D, Windt E, Buske A, Albrecht B, Prott EC, Seemanová E, Seidel J, Rosenbaum T, Jenne D, Kehrer-Sawatzki H, Tinschert S (2005) Childhood overgrowth in patients with common NF1 microdeletions. Eur J Hum Genet 13:883–888
Koczkowska M, Chen Y, Callens T, Gomes A, Sharp A, Johnson S, Hsiao MC, Chen Z, Balasubramanian M, Barnett CP, Becker TA, Ben-Shachar S, Bertola DR, Blakeley JO, Burkitt-Wright EMM, Callaway A, Crenshaw M, Cunha KS, Cunningham M, D’Agostino MD, Dahan K, De Luca A, Destrée A, Dhamija R, Eoli M, Evans DGR, Galvin-Parton P, George-Abraham JK, Gripp KW, Guevara-Campos J, Hanchard NA, Hernández-Chico C, Immken L, Janssens S, Jones KJ, Keena BA, Kochhar A, Liebelt J, Martir-Negron A, Mahoney MJ, Maystadt I, McDougall C, McEntagart M, Mendelsohn N, Miller DT, Mortier G, Morton J, Pappas J, Plotkin SR, Pond D, Rosenbaum K, Rubin K, Russell L, Rutledge LS, Saletti V, Schonberg R, Schreiber A, Seidel M, Siqveland E, Stockton DW, Trevisson E, Ullrich NJ, Upadhyaya M, van Minkelen R, Verhelst H, Wallace MR, Yap YS, Zackai E, Zonana J, Zurcher V, Claes K, Martin Y, Korf BR, Legius E, Messiaen LM (2018)Genotype-phenotype correlation in NF1: evidence for a more severe phenotype associated with missense mutations affecting NF1 codons 844-848. Am J Hum Genet 102:69–87
Koczkowska M, Callens T, Gomes A, Sharp A, Chen Y, Hicks AD, Aylsworth AS, Azizi AA, Basel DG, Bellus G, Bird LM, Blazo MA, Burke LW, Cannon A, Collins F, De Filippo C, Denayer E, Digilio MC, Dills SK, Dosa L, Greenwood RS, Griffis C, Gupta P, Hachen RK, Hernández-Chico C, Janssens S, Jones KJ, Jordan JT, Kannu P, Korf BR, Lewis AM, Listernick RH, Lonardo F, Mahoney MJ, Ojeda MM, McDonald MT, McDougall C, Mendelsohn N, Miller DT, Mori M, Oostenbrink R, Perreault S, Pierpont ME, Piscopo C, Pond DA, Randolph LM, Rauen KA, Rednam S, Rutledge SL, Saletti V, Schaefer GB, Schorry EK, Scott DA, Shugar A, Siqveland E, Starr LJ, Syed A, Trapane PL, Ullrich NJ, Wakefield EG, Walsh LE, Wangler MF, Zackai E, KBM C, Wimmer K, van Minkelen R, De Luca A, Martin Y, Legius E, Messiaen LM (2019) Expanding the clinical phenotype of individuals with a 3-bp in-frame deletion of the NF1 gene (c.2970_2972del): an update of genotype-phenotype correlation. Genet Med 21:867–876
Rojnueangnit K, Xie J, Gomes A, Sharp A, Callens T, Chen Y, Liu Y, Cochran M, Abbott MA, Atkin J, Babovic-Vuksanovic D, Barnett CP, Crenshaw M, Bartholomew DW, Basel L, Bellus G, Ben-Shachar S, Bialer MG, Bick D, Blumberg B, Cortes F, David KL, Destree A, Duat-Rodriguez A, Earl D, Escobar L, Eswara M, Ezquieta B, Frayling IM, Frydman M, Gardner K, Gripp KW, Hernández-Chico C, Heyrman K, Ibrahim J, Janssens S, Keena BA, Llano-Rivas I, Leppig K, McDonald M, Misra VK, Mulbury J, Narayanan V, Orenstein N, Galvin-Parton P, Pedro H, Pivnick EK, Powell CM, Randolph L, Raskin S, Rosell J, Rubin K, Seashore M, Schaaf CP, Scheuerle A, Schultz M, Schorry E, Schnur R, Siqveland E, Tkachuk A, Tonsgard J, Upadhyaya M, Verma IC, Wallace S, Williams C, Zackai E, Zonana J, Lazaro C, Claes K, Korf B, Martin Y, Legius E, Messiaen L (2015) High incidence of Noonan syndrome features including short stature and pulmonic stenosis in patients carrying NF1 missense mutations affecting p.Arg1809: genotype-phenotype correlation. Hum Mutat 36:1052–1063
Upadhyaya M, Huson SM, Davies M, Thomas N, Chuzhanova N, Giovannini S, Evans DG, Howard E, Kerr B, Griffiths S, Consoli C, Side L, Adams D, Pierpont M, Hachen R, Barnicoat A, Li H, Wallace P, Van Biervliet JP, Stevenson D, Viskochil D, Baralle D, Haan E, Riccardi V, Turnpenny P, Lazaro C, Messiaen L (2007) An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): evidence of a clinically significant NF1 genotype-phenotype correlation. Am J Hum Genet 80:140–151
Pinna V, Lanari V, Daniele P, Consoli F, Agolini E, Margiotti K, Bottillo I, Torrente I, Bruselles A, Fusilli C, Ficcadenti A, Bargiacchi S, Trevisson E, Forzan M, Giustini S, Leoni C, Zampino G, Digilio MC, Dallapiccola B, Clementi M, Tartaglia M, De Luca A (2015) p.Arg1809Cys substitution in neurofibromin is associated with a distinctive NF1 phenotype without neurofibromas. Eur J Hum Genet 23:1068–1071
Evans DG, Hartley CL, Smith PT, King AT, Bowers NL, Tobi S, Wallace AJ, Perry M, Anup R, Lloyd SKW, Rutherford SA, Hammerbeck-Ward C, Pathmanaban ON, Stapleton E, Freeman SR, Kellett M, Halliday D, Parry A, Gair JJ, Axon P, Laitt R, Thomas O, Afridi SK, Obholzer R, Duff C, Stivaros SM, Vassallo G, Harkness EF, Smith MJ, group ESNr (2020) Incidence of mosaicism in 1055 de novo NF2 cases: much higher than previous estimates with high utility of next-generation sequencing. Genet Med 22:53–59
Payne JM, Haebich KM, MacKenzie R, Walsh KS, Hearps SJC, Coghill D, Barton B, Pride NA, Ullrich NJ, Tonsgard JH, Viskochil D, Schorry EK, Klesse L, Fisher MJ, Gutmann DH, Rosser T, Packer RJ, Korf B, Acosta MT, Bellgrove MA, North KN (2019) Cognition, ADHD symptoms, and functional impairment in children and adolescents with neurofibromatosis type 1. J Atten Disord:1087054719894384
Morris SM, Acosta MT, Garg S, Green J, Huson S, Legius E, North KN, Payne JM, Plasschaert E, Frazier TW, Weiss LA, Zhang Y, Gutmann DH, Constantino JN (2016) Disease burden and symptom structure of autism in neurofibromatosis type 1: a study of the International NF1-ASD Consortium Team (INFACT). JAMA Psychiatry 73:1276–1284
Ottenhoff MJ, Rietman AB, Mous SE, Plasschaert E, Gawehns D, Brems H, Oostenbrink R, van Minkelen R, Nellist M, Schorry E, Legius E, Moll HA, Elgersma Y, Team E-N(2020) Examination of the genetic factors underlying the cognitive variability associated with neurofibromatosis type 1. Genet Med 22:889–897
Barba C, Jacques T, Kahane P, Polster T, Isnard J, Leijten FS, Ozkara C, Tassi L, Giordano F, Castagna M, John A, Oz B, Salon C, Streichenberger N, Cross JH, Guerrini R (2013) Epilepsy surgery in neurofibromatosis type 1. Epilepsy Res 105:384–395
Nix JS, Blakeley J, Rodriguez FJ (2020) An update on the central nervous system manifestations of neurofibromatosis type 1. Acta Neuropathol 139:625–641
Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, Banerjee A, Packer RJ, Kilburn LB, Goldman S, Pollack IF, Qaddoumi I, Jakacki RI, Fisher PG, Dhall G, Baxter P, Kreissman SG, Stewart CF, Jones DTW, Pfister SM, Vezina G, Stern JS, Panigrahy A, Patay Z, Tamrazi B, Jones JY, Haque SS, Enterline DS, Cha S, Fisher MJ, Doyle LA, Smith M, Dunkel IJ, Fouladi M (2019) Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 20:1011–1022
Listernick R, Darling C, Greenwald M, Strauss L, Charrow J (1995) Optic pathway tumors in children: the effect of neurofibromatosis type 1 on clinical manifestations and natural history. J Pediatr 127:718–722
Listernick R, Ferner RE, Piersall L, Sharif S, Gutmann DH, Charrow J (2004)Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology 63:1944–1946
Santoro C, Perrotta S, Picariello S, Scilipoti M, Cirillo M, Quaglietta L, Cinalli G, Cioffi D, Di Iorgi N, Maghnie M, Gallizia A, Parpagnoli M, Messa F, De Sanctis L, Vannelli S, Marzuillo P, Miraglia Del Giudice E, Grandone A (2020) Pretreatment endocrine disorders due to optic pathway gliomas in pediatric neurofibromatosis type 1: multicenter study. J Clin Endocrinol Metab 105
Caen S, Cassiman C, Legius E, Casteels I (2015) Comparative study of the ophthalmological examinations in neurofibromatosis type 1. Proposal for a new screening algorithm. Eur J Paediatr Neurol 19:415–422
D’Angelo F, Ceccarelli M, Tala GL, Zhang J, Frattini V, Caruso FP, Lewis G, Alfaro KD, Bauchet L, Berzero G, Cachia D, Cangiano M, Capelle L, de Groot J, DiMeco F, Ducray F, Farah W, Finocchiaro G, Goutagny S, Kamiya-Matsuoka C, Lavarino C, Loiseau H, Lorgis V, Marras CE, McCutcheon I, Nam DH, Ronchi S, Saletti V, Seizeur R, Slopis J, Suñol M, Vandenbos F, Varlet P, Vidaud D, Watts C, Tabar V, Reuss DE, Kim SK, Meyronet D, Mokhtari K, Salvador H, Bhat KP, Eoli M, Sanson M, Lasorella A, Iavarone A (2019) The molecular landscape of glioma in patients with neurofibromatosis 1. Nat Med 25:176–187
Terry AR, Jordan JT, Schwamm L, Plotkin SR (2016) Increased risk of cerebrovascular disease among patients with neurofibromatosis type 1: population-based approach. Stroke 47:60–65
Rea D, Brandsema JF, Armstrong D, Parkin PC, deVeber G, MacGregor D, Logan WJ, Askalan R (2009) Cerebral arteriopathy in children with neurofibromatosis type 1. Pediatrics 124:e476–e483
Cairns AG, North KN (2008) Cerebrovascular dysplasia in neurofibromatosis type 1. J Neurol Neurosurg Psychiatry 79:1165–1170
Guillamo JS, Créange A, Kalifa C, Grill J, Rodriguez D, Doz F, Barbarot S, Zerah M, Sanson M, Bastuji-Garin S, Wolkenstein P, France RN (2003) Prognostic factors of CNS tumours in neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain 126:152–160
Rasmussen SA, Yang Q, Friedman JM (2001) Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet 68:1110–1118
Griffiths PD, Blaser S, Mukonoweshuro W, Armstrong D, Milo-Mason G, Cheung S (1999) Neurofibromatosis bright objects in children with neurofibromatosis type 1: a proliferative potential? Pediatrics 104:e49
Billiet T, Mädler B, D’Arco F, Peeters R, Deprez S, Plasschaert E, Leemans A, Zhang H, den Bergh BV, Vandenbulcke M, Legius E, Sunaert S, Emsell L (2014) Characterizing the microstructural basis of “unidentified bright objects” in neurofibromatosis type 1: a combined in vivo multicomponent T2 relaxation and multi-shell diffusion MRI analysis. Neuroimage Clin 4:649–658
Moore BD, Slopis JM, Jackson EF, De Winter AE, Leeds NE (2000) Brain volume in children with neurofibromatosis type 1: relation to neuropsychological status. Neurology 54:914–920
Nguyen R, Jett K, Harris GJ, Cai W, Friedman JM, Mautner VF (2014) Benign whole body tumor volume is a risk factor for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. J Neuro-Oncol 116:307–313
Miao R, Wang H, Jacobson A, Lietz AP, Choy E, Raskin KA, Schwab JH, Deshpande V, Nielsen GP, DeLaney TF, Cote GM, Hornicek FJ, Chen YE (2019)Radiation-induced and neurofibromatosis-associated malignant peripheral nerve sheath tumors (MPNST) have worse outcomes than sporadic MPNST. Radiother Oncol 137:61–70
Yamanaka R, Hayano A (2017)Radiation-induced malignant peripheral nerve sheath tumors: a systematic review. World Neurosurg 105:961-970.e968
Malbari F, Spira M, Knight PB, Zhu C, Roth M, Gill J, Abbott R, Levy AS (2018) Malignant peripheral nerve sheath tumors in neurofibromatosis: impact of family history. J Pediatr Hematol Oncol 40:e359–e363
Brems H, Chmara M, Sahbatou M, Denayer E, Taniguchi K, Kato R, Somers R, Messiaen L, De Schepper S, Fryns J-P, Cools J, Marynen P, Thomas G, Yoshimura A, Legius E (2007) Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat Genet 39:1120–1126
Messiaen L, Yao S, Brems H, Callens T, Sathienkijkanchai A, Denayer E, Spencer E, Arn P, Babovic-Vuksanovic D, Bay C, Bobele G, Cohen BH, Escobar L, Eunpu D, Grebe T, Greenstein R, Hachen R, Irons M, Kronn D, Lemire E, Leppig K, Lim C, McDonald M, Narayanan V, Pearn A, Pedersen R, Powell B, Shapiro LR, Skidmore D, Tegay D, Thiese H, Zackai EH, Vijzelaar R, Taniguchi K, Ayada T, Okamoto F, Yoshimura A, Parret A, Korf B, Legius E (2009) Clinical and mutational spectrum of neurofibromatosis type 1-like syndrome. Jama-Journal of the American Medical Association 302:2111–2118
Pasmant E, Parfait B, Luscan A, Goussard P, Briand-Suleau A, Laurendeau I, Fouveaut C, Leroy C, Montadert A, Wolkenstein P, Vidaud M, Vidaud D (2015) Neurofibromatosis type 1 molecular diagnosis: what can NGS do for you when you have a large gene with loss of function mutations? Eur J Hum Genet 23:596–601
Dunzendorfer-Matt T, Mercado EL, Maly K, McCormick F, Scheffzek K (2016) The neurofibromin recruitment factor Spred1 binds to the GAP related domain without affecting Ras inactivation. Proc Natl Acad Sci U S A 113:7497–7502
Hirata Y, Brems H, Suzuki M, Kanamori M, Okada M, Morita R, Llano-Rivas I, Ose T, Messiaen L, Legius E, Yoshimura A (2016) Interaction between a domain of the negative regulator of the Ras-ERK pathway, SPRED1 protein, and the GTPase-activating protein-related domain of neurofibromin is implicated in Legius syndrome and neurofibromatosis type 1. J Biol Chem 291:3124–3134
Stowe IB, Mercado EL, Stowe TR, Bell EL, Oses-Prieto JA, Hernández H, Burlingame AL, McCormick F (2012) A shared molecular mechanism underlies the human rasopathies Legius syndrome and neurofibromatosis 1. Genes Dev 26:1421–1426
Yoshimura A (2009) Regulation of cytokine signaling by the SOCS and Spred family proteins. Keio J Med 58:73–83
Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns J (2002) PTPN11 mutations in LEOPARD syndrome. J Med Genet 39:571–574
Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, Pizzuti A, Dallapiccola B (2002) Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet 71:389–394
Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, López Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD (2007)Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet 39:1007–1012
Conboy E, Dhamija R, Wang M, Xie J, Dyck PJ, Bridges AG, Spinner RJ, Clayton AC, Watson RE, Messiaen L, Babovic-Vuksanovic D (2016) Paraspinal neurofibromas and hypertrophic neuropathy in Noonan syndrome with multiple lentigines. J Med Genet 53:123–126
Aoki Y, Niihori T, Inoue S, Matsubara Y (2016) Recent advances in RASopathies. J Hum Genet 61:33–39
Oishi K, Zhang H, Gault WJ, Wang CJ, Tan CC, Kim IK, Ying H, Rahman T, Pica N, Tartaglia M, Mlodzik M, Gelb BD (2009)Phosphatase-defective LEOPARD syndrome mutations in PTPN11 gene have gain-of-function effects during Drosophila development. Hum Mol Genet 18:193–201
Suerink M, Ripperger T, Messiaen L, Menko FH, Bourdeaut F, Colas C, Jongmans M, Goldberg Y, Nielsen M, Muleris M, van Kouwen M, Slavc I, Kratz C, Vasen HF, Brugiѐres L, Legius E, Wimmer K (2019) Constitutional mismatch repair deficiency as a differential diagnosis of neurofibromatosis type 1: consensus guidelines for testing a child without malignancy. J Med Genet 56:53–62
Wimmer K, Kratz CP, Vasen HF, Caron O, Colas C, Entz-Werle N, Gerdes AM, Goldberg Y, Ilencikova D, Muleris M, Duval A, Lavoine N, Ruiz-Ponte C, Slavc I, Burkhardt B, Brugieres L, (C4CMMRD)E-CCfC(2014) Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium ‘care for CMMRD’ (C4CMMRD). J Med Genet 51:355–365
Wimmer K, Rosenbaum T, Messiaen L (2017) Connections between constitutional mismatch repair deficiency syndrome and neurofibromatosis type 1. Clin Genet 91:507–519
Spritz R (2011) Letter: Misdiagnosis of “neurofibromatosis” in patients with piebaldism. Dermatol Online J 17:13
Stevens CA, Chiang PW, Messiaen LM (2012) Café-au-lait macules and intertriginous freckling in piebaldism: clinical overlap with neurofibromatosis type 1 and Legius syndrome. Am J Med Genet A 158A:1195–1199
Romanet P, Philibert P, Fina F, Cuny T, Roche C, Ouafik L, Paris F, Reynaud R, Barlier A (2019) Using digital droplet polymerase chain reaction to detect the mosaic GNAS mutations in whole blood DNA or circulating cell-free DNA in fibrous dysplasia and McCune-Albright syndrome. J Pediatr 205:281-285.e284
Spencer T, Pan KS, Collins MT, Boyce AM (2019) The clinical spectrum of McCune-Albright syndrome and its management. Horm Res Paediatr:1–10
Halliday D, Parry A, Evans DG (2019) Neurofibromatosis type 2 and related disorders. Curr Opin Oncol 31:562–567
Hulsebos TJ, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P (2007) Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet 80:805–810
Piotrowski A, Xie J, Liu YF, Poplawski AB, Gomes AR, Madanecki P, Fu C, Crowley MR, Crossman DK, Armstrong L, Babovic-Vuksanovic D, Bergner A, Blakeley JO, Blumenthal AL, Daniels MS, Feit H, Gardner K, Hurst S, Kobelka C, Lee C, Nagy R, Rauen KA, Slopis JM, Suwannarat P, Westman JA, Zanko A, Korf BR, Messiaen LM (2014) Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat Genet 46:182–187
Steklov M, Pandolfi S, Baietti MF, Batiuk A, Carai P, Najm P, Zhang M, Jang H, Renzi F, Cai Y, Abbasi Asbagh L, Pastor T, De Troyer M, Simicek M, Radaelli E, Brems H, Legius E, Tavernier J, Gevaert K, Impens F, Messiaen L, Nussinov R, Heymans S, Eyckerman S, Sablina AA (2018) Mutations in LZTR1 drive human disease by dysregulating RAS ubiquitination. Science 362:1177–1182
Louvrier C, Pasmant E, Briand-Suleau A, Cohen J, Nitschké P, Nectoux J, Orhant L, Zordan C, Goizet C, Goutagny S, Lallemand D, Vidaud M, Vidaud D, Kalamarides M, Parfait B (2018) Targeted next-generation sequencing for differential diagnosis of neurofibromatosis type 2, schwannomatosis, and meningiomatosis. Neuro-Oncology 20:917–929
Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, Whitcomb P, Martin S, Aschbacher-Smith LE, Rizvi TA, Wu J, Ershler R, Wolters P, Therrien J, Glod J, Belasco JB, Schorry E, Brofferio A, Starosta AJ, Gillespie A, Doyle AL, Ratner N, Widemann BC (2016) Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med 375:2550–2560
Gross AM, Wolters PL, Dombi E, Baldwin A, Whitcomb P, Fisher MJ, Weiss B, Kim A, Bornhorst M, Shah AC, Martin S, Roderick MC, Pichard DC, Carbonell A, Paul SM, Therrien J, Kapustina O, Heisey K, Clapp DW, Zhang C, Peer CJ, Figg WD, Smith M, Glod J, Blakeley JO, Steinberg SM, Venzon DJ, Doyle LA, Widemann BC (2020) Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 382:1430–1442
Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Young Poussaint T, Fangusaro J, Phillips J, Perry A, Turner D, Prados M, Packer RJ, Qaddoumi I, Gururangan S, Pollack IF, Goldman S, Doyle LA, Stewart CF, Boyett JM, Kun LE, Fouladi M (2017) A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro-Oncology 19:1135–1144
Perreault S, Larouche V, Tabori U, Hawkin C, Lippé S, Ellezam B, Décarie JC, Théoret Y, Métras M, Sultan S, Cantin É, Routhier M, Caru M, Legault G, Bouffet É, Lafay-Cousin L, Hukin J, Erker C, Jabado N (2019) A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer 19:1250
Klesse LJ, Jordan JT, Radtke HB, Rosser T, Schorry E, Ullrich N, Viskochil D, Knight P, Plotkin SR, Yohay K (2020) The use of MEK inhibitors in neurofibromatosis type 1-associated tumors and management of toxicities. Oncologist
Packer RJ, Iavarone A, Jones DTW, Blakeley JO, Bouffet E, Fisher MJ, Hwang E, Hawkins C, Kilburn L, MacDonald T, Pfister SM, Rood B, Rodriguez FJ, Tabori U, Ramaswamy V, Zhu Y, Fangusaro J, Johnston SA, Gutmann DH (2020) Implications of new understandings of gliomas in children and adults with NF1: report of a consensus conference. Neuro-Oncology 22:773–784
Author information
Authors and Affiliations
Contributions
Both authors were involved in the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Eric Legius received consulting fees from AstraZeneca and SpringWorks Therapeutics.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Legius, E., Brems, H. Genetic basis of neurofibromatosis type 1 and related conditions, including mosaicism. Childs Nerv Syst 36, 2285–2295 (2020). https://doi.org/10.1007/s00381-020-04771-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04771-8