Abstract
Background
Myelomeningocoele (MMC) is common in the developing world. The purpose of this study was to investigate the clinical characteristics and management of myelomeningocoele and to identify factors contributing to outcomes.
Methods
This was a retrospective, observational study of consecutive children diagnosed with MMC managed in the Paediatric Neurosurgery Unit at Inkosi Albert Luthuli Central Hospital. Multiple logistic regression analysis identified clinical characteristics, demographics and surgical variables that were associated with outcome.
Results
A total of 309 children were managed during this period (M:F 1.3:1). The most common sites were lumbar, lumbo-sacral and sacral. Mean age at surgical repair was 4.7 ± 15.6 months. Two hundred and eight children had ventriculomegaly, of whom 158 had symptomatic hydrocephalus, requiring CSF diversion. Fifty-eight (21%) patients developed wound sepsis, of whom 13 (22%) developed meningitis (p = 0.001). The time to wound sepsis was 9.5 ± 3.6 days. The commonest organism isolated was Staphylococcus aureus followed by MRSA. Thirty-two patients (23%) developed shunt malfunction and three (11%) developed ETV malfunction. Twenty children (9%) demised during the admission period. Death was associated with meningitis (p < 0.0001), and meningitis itself was associated with wound sepsis (p < 0.0001). Hospital stay was 20.4 ± 16 days. Wound sepsis (p = 0.002) and meningitis (p < 0.0001), respectively, were associated with prolonged hospital stay.
Conclusion
There was a slight male preponderance and hydrocephalus occurred in two thirds of cases. Wound sepsis and meningitis were associated poor outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neural tube defects (NTDs) result from failure of neural tube closure that normally occurs at 15–28 days after conception [1]. Myelomeningocele (MMC) is by far the most common and devastating form of NTD, which comprises a sac-like structure that protudes through the vertebral arches and contains meninges, cerebrospinal fluid (CSF) and spinal cord [1, 2].
The incidence of NTD in South Africa (SA) is reported to be 0.77–6.1 per 1000 live births [1, 2], which is slightly higher than the international incidence of 0.17–6.39 per 1000 live births [2,3,4,5]. In East Africa, the prevalence of NTDs is estimated to be 1.3 cases per 1000 live births [6]. There are approximately 300,000 new cases of NTDs annually, and 40,000 of these cases were estimated to occur in sub-Saharan Africa [6]. This variation is influenced by racial, geographic and socioeconomic status of the regions surveyed. The incidence of NTDs has previously been under-reported in sub-Saharan Africa due to lack of awareness on the part of parents and limited access to healthcare facilities [6].
The diagnosis of NTD is life-changing for the child, the parents and the community [7]. The condition is associated with CNS abnormalities, resulting in disabilities such as sensory, motor, bladder and bowel dysfunction together with orthopaedic deformities and learning disabilities which can shorten the child’s life-span, and have psychosocial implications for the child including integration into their communities [8]. The optimal management requires a multidisciplinary effort which can be challenging in a resource-limited environment [7].
The literature on MMC is sparse in developing countries like South Africa, which are predisposed to the development of birth defects. The purpose of this paper was to report on clinical characteristics, management and post-surgical outcomes of children diagnosed with MMC in the KwaZulu-Natal (KZN) Province of South Africa. This was achieved by a retrospective observational study of MMC in a tertiary neurosurgical unit based in a developing country.
Methods and materials
This was a retrospective study of consecutive children with the diagnosis of MMC admitted into the Paediatric Unit in the Department of Neurosurgery at Inkosi Albert Luthuli Central Hospital (IALCH), a tertiary institution located in Durban, the main coastal city of the KZN Province. The Province is located on the east coast of South Africa and has a surface area of 94,361 km2 [9].. It has a population of 10 million people of which 3.5 million are under the age of 12 years. Our unit provides the only paediatric neurosurgery service for this Province.
We reviewed the charts of all children with a diagnosis of myelomeningocoele admitted between January 2006 and December 2014. Patient data are kept in a password-protected hospital information management system (Soarian ®, Siemens, USA). The data were analysed for variables such as mode of delivery, demographic profile, clinical presentation, MMC location, presence of CSF leak, age at MMC repair, associated hydrocephalus and management thereof including septic complications associated with MMC repair. The study also assessed the method of CSF diversion and their outcomes as well as length of hospital stay and in-hospital mortality. We excluded patients with incomplete information in their medical files and those who were treated in neurosurgery units outside IALCH. This study was granted approval by the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal.
The repair of the MMC in our unit involves dissection and reconstruction of the neural placode protecting any functional nerve roots. This is followed by closure of the dura and tension-free closure of myofascial tissue. Complex defects that require flap reconstruction are closed in conjunction with the plastic surgeons. The indication for the insertion of VPS is the presence of active hydrocephalus (HCP). As a policy, we do not insert VP shunts at the same surgical operation as repair of MMC. Patients who developed symptomatic hydrocephalus and were suitable candidates were offered endoscopic third ventriculostomy (ETV).
Statistical analysis was performed using SPSS 21 (Chicago, Illinois, United States of America). Mean and standard deviation was used for categorical variables. Student t test and Mann-Whitney test were used for continuous variables. We assessed the association between time to surgery, presentation with CSF leak and hydrocephalus with wound sepsis and mortality. A p value of less than 0.05 was considered statistically significant.
Results
A total of 314 children were treated for MMC at our neurosurgical unit during the study period. Five were excluded because of incomplete information in the files, leaving 309 children for analysis. Figure 1 shows geographical distribution of the referral patern of MMC in KZN. There were 173 males (M:F ratio 1.3:1). One hundred and thirty-five children (44%) were delivered via caesarean section and the rest by normal vaginal delivery. Antenatal diagnosis of MMC was possible in only six children (1.9%). Maternal age was documented in only 129 files. Of these,7 mothers (2.3%) were < 18 years of age, 104 were 18–35 years old (33.7%) and 18 (14%) were > 35 years. Only 240 mothers (78%) had attended antenatal clinic; 23 did not attend antenatal clinic (7%) and antenatal clinic attendance was not documented in 46 mothers (15%). There was a family history of MMC in only two mothers; one mother had an older child with MMC and another reported one family member mothering a child with MMC. The majority of MMC were in the lumbar region followed by the lumbo-sacral spine (Table 1). There was CSF leak from the lesion in 156 children (51%) mainly from the lumbar area (Table 1) and 141 children (48%) presented with complete paralysis of the lower limbs.
Mean age at surgical repair was 4.7 ± 15.6 months. Flap reconstruction was required in 20 children (7.9%). Radiological evidence of ventriculomegaly was seen in 208 children (67%) and symptomatic HCP was observed in only 145 of these (70%). CSF diversion with VPS was necessary in 144 children. ETV was performed as an initial treatment option in one child.
Thirty-two of the 144 patients who underwent VPS (22%) developed shunt malfunction (Table 2). The time to shunt malfunction was 176 ± 83.3 days. Infection was responsible for shunt malfunction in 15 of the 32 children (47%). Nineteen of the 32 children with shunt malfunction underwent revision of the blocked shunt and the other thirteen underwent ETV. In total, therefore, 14 patients underwent ETV. ETV malfunction occurred in three patients after a median 31 days (30–122), all of whom were subsequently managed with VPS insertion; ETV was not reattempted.
Fifty-eight (21%) children developed postoperative wound sepsis, of whom 35 (60%) required surgical debridement, while chemical debridement was sufficient in the rest. The time to wound sepsis was 9.5 ± 3.6 days. The most common organism responsible for sepsis was Staphylococcus aureus (Table 3). The presence CSF leak (p = 0.20) and/or hydrocephalus (p = 0.39) were not independently associated with wound infection. Thirty-four children developed meningitis in the postoperative period (11%).
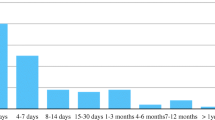
Figure 2 shows the length of hospital stay in children with and without wound sepsis, and Fig. 3 shows comparison of length of hopital stay in children with and without meningitis. Hospital stay was longer for patients with wound sepsis and meningitis. Twenty children (9%) demised during the admission period. Factors influencing mortality, sepsis and meningitis are shown in Tables 4, 5 and 6, respectively. Wound sepsis played a significant role in influencing meningitis (p = 0.002), and meningitis was itself a significant factor in influencing mortality (p < 0.001). The need for flap reconstruction significantly influenced wound sepsis (p < 0.001). Only 54 patients were available for follow-up with the mean follow-up period being 24 + 16.42 months. One patient is known to have died during follow-up.
Discussion
Observations made from this study are similar to those in the international literature with a few exceptions. There was a slight but not clinically significant male preponderance. The occurrence of the MMC sac in the lumbar, lumbo-sacral and sacral areas is in keeping with the international literature where the common sites of occurrence are the lumbar and lumbosacral areas [10,11,12]. HCP was noted in 67% in this series which falls within the 51–80% reported in the literature [4, 13,14,15,16].
Surgical repair of the MMC is generally performed within 24 h after birth to minimize the risk of infection and to preserve existing function in the spinal cord [7]. Most authors [11, 17] concur that a surgical closure longer than 48 h after delivery is an important risk factor for wound sepsis and other complications, while others contend that the timing of MMC repair has no influence on complications [15,16,17]. In our setting, patients are referred for repair long after birth, which explains the late age of repair of 4.7 months in this study.
The delay in surgical repair of these lesions is not ideal or standard of care. The constraints of healthcare resources are the key factors resulting in these undesirable delays in our setting. In children with delayed presentation, the neural placode is destroyed and overgrown by granulation tissue. At surgical repair, the granulation tissue is resected, the dura is closed in watertight fashion and the myofascial tissue is mobilized to achieve a tension-free closure [18]. One cannot determine with certainty whether any neural tissue is functional at this stage, but the principle is to preserve identifiable neural tissue [18].
VPS insertion remains the gold standard for the treatment of HCP in children with MMC. However, it is not without complications. It is known to have a significant failure rate, particularly related to infective complications [10, 19]. Furthermore, these children already have vulnerable CNS tissue and the development of additional infection further worsens cognitive function and other outcomes. This has made the timing of VPS insertion an area of debate [19]. Whereas some authors suggest that VPS insertion at the same time or within a week of myelomeningocele closure increases the risk of shunt infection [10, 15, 20], others report no significant difference in the rate of shunt infection and dysfunction [21]. Yet other studies report that a delay in MMC repair surgery significantly increases the risk for ventriculitis and meningitis [22]. Our Unit has adopted a policy of employing delayed insertion of VP shunt, especially in the presence of a leaking MMC, the rationale being to limit the rate of infection.
Surgical site infection is a frequent complication in myelomeningocele repair [12]. The prevalence of wound sepsis in this series was 21% which falls within the 12.8–34% reported in the literature [11, 12, 16, 23]. Risk factors for surgical site infection are delayed presentation, size of defect, length of operative procedure and the use of flap reconstruction [11, 12]. Similarly in this study delayed presentation and use of surgical flap reconstruction contributed towards a high rate of surgical site infection. Although the source of sepsis following MMC repair varies in the literature [12], the profile of microorganisms isolated in our patients are similar to other reported series [11, 12, 24]. Demir et al. noted that cultured microorganisms in children with MMC were of nosocomial origin and resistant to antibiotics [11].
Shunt malfunction developed in 22% in this series which falls within the 15–45.9% reported in the literature [25,26,27]. The common causes of shunt malfunction are shunt blockade (11–20%) [11, 16, 26, 27], shunt infection (3.5%) and shunt migration (0.9%) [26, 27]. Risk factors for the development of meningitis include CSF leakage from the MMC, and complex defects requiring flap transposition [11, 12, 16]. The 11% meningitis rate in this series was lower than the reported 19–21% [11].
Our in-hospital mortality rate was 9%. This is higher than the 5–7.5% mortality rate reported by in the international literature [10, 28]. This study also showed meningitis and wound sepsis to be significant factors influencing mortality and length of hospital stay. The need for flap reconstruction also indirectly contributed to increased hospital stay. This is supported by Demir et al., who have shown that meningitis, VP shunt infection and surgical wound-site infection prolong the duration of hospitalization and increase hospital costs [11].
The advent of in-utero repair of MMC [29] is a welcome development. Where fetal surgery is considered, prenatal evaluation should include high resolution ultrasound, magnetic resonance imaging and amniocentesis [30]. However, this form of treatment is not routinely performed in developing counties [3]. Only 2% of MMC were diagnosed in the prenatal stage; this is despite 78% of the mothers having attended antenatal care clinics. This is in contrast to the findings in the world literature where antenatal diagnosis can be as high as 60% [31]. This low rate of prenatal diagnosis may be explained by the lack of screening tools and expertise in most antenatal care centres in our setting. This leaves many of these congenital defects undetected until delivery. Furthermore, performing an anatomical scan at 18–23 weeks requires appropriate training in the use of a 3D scanner [2]. Such resource is only available in IALCH,which is the only referral centre for high-risk maternal cases. We are in agreement with other authors that the late or no presentation for antenatal care and the low antenatal diagnosis rate in developing countries may be related to the lack of awareness of the population regarding this condition [32, 33].
The South African Government has put emphasis on primary healthcare, which include antenatal care clinics [29]. This will allow in utero diagnosis of NTDs which should be accompanied by counselling with regard to fetal surgery or termination of pregnancy as provided for by the Choice on Termination of Pregnancy Act No. 92 of 1996 [34]. Despite advances in the treatment of MMC, no treatment exists that will completely eliminate the serious disability or premature mortality associated with it. Thus managing children with MMC in sub-Saharan Africa can be potentially devastating due to prevailing low socioeconomic status, inadequate health facilities and other factors [35].
This study does have some limitations. These include the fact that the design was retrospective, and not all data were available for analysis. However, it reflects a large number of the burden of MMC in a South African institution serving a large population.
In conclusion, myelomeningocele is one of the main contributors of burden of disease affecting children managed in a Neurosurgical unit in KZN. There was a slight male preponderance and hydrocephalus was present in two thirds of cares. Myelomeningocoele was associated with significant morbidity. Wound sepsis and meningitis were associated with prolongation of hospital stay. The need for flap reconstruction together with resultant wound sepsis as well as meningitis were associated poor outcomes.
References
Fieggen K, Stewart C (2014) Aetiology and antenatal diagnosis of spina bifida. S Afr Med J 104(3):218
Fieggen G, Fieggen K, Stewart C et al (2014) Spina bifida: a multidisciplinary perspective on a many-faceted condition. S Afr Med J 104(3):212–217
Adzick NS, Thom EA, Spong CY, Brock JW 3rd, Burrows PK, Johnson MP, Howell LJ, Farrell JA, Dabrowiak ME, Sutton LN, Gupta N, Tulipan NB, D'Alton ME, Farmer DL, MOMS Investigators (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364(11):993–1004
Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA (2001) Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg 34(3):114–120
Mulinare J, Cordero JF, Erickson JD, Berry RJ (1988) Periconceptional use of multivitamins and the occurrence of neural tube defects. Jama 260(21):3141–3145
Githuku JN, Azofeifa A, Valencia D et al (2014) Assessing the prevalence of spina bifida and encephalocele in a Kenyan hospital from 2005–2010: implications for a neural tube defects surveillance system. Pan Afr Med J 18:60
Yi Y, Lindemann M, Colligs A, Snowball C (2011) Economic burden of neural tube defects and impact of prevention with folic acid: a literature review. Eur J Pediatr 170(11):1391–1400
Flores A, Vellozzi C, Valencia D, Sniezek J (2014) Global burden of neural tube defects, risk factors, and prevention. Indian J Community Health 26(Suppl 1):3
Statistics SA. Census 2011, Statistics South Africa, 2012
Arslan M, Eseoglu M, Gudu BO et al (2011) Comparison of simultaneous shunting to delayed shunting in infants with myelomeningocele in terms of shunt infection rate. Turk Neurosurg 21(3):397–402
Demir N, Peker E, Gülşen İ, Ağengin K, Tuncer O (2015) Factors affecting infection development after meningomyelocele repair in newborns and the efficacy of antibiotic prophylaxis. Childs Nerv Syst 31(8):1355–1359
Neves N, Noleto M, Ribeiro V (2017) Prevalence of and risk factors for surgical site infections in patients with myelomeningocoele. Revista SOBECC 22(1):10–16
Crimmins D, Hayward R, Thompson D (2005) Reducing shunt placement rate in myelomeningocele patients (abstr). Childs Nerv Syst 21:828–829
de la Cruz R, Millán JM, Miralles M, Muñoz MJ (1989) Cranial sonographic evaluation in children with meningomyelocele. Childs Nerv Syst 5(2):94–98
Machado HR, de Oliveira RS (2004) Simultaneous repair of myelomeningocele and shunt insertion. Childs Nerv Syst 20(2):107–109
Schroeder HK, Nunes JC, Madeira L, Moritz JLW, Walz R, Linhares MN (2012) Postsurgical infection after myelomeningocele repair: a multivariate analysis of 60 consecutive cases. Clin Neurol Neurosurg 114(7):981–985
Marreiros H, Loff C, Calado E (2015) Who needs surgery for pediatric myelomeningocele? A retrospective study and literature review. J Spinal Cord Med 38(5):626–640
Padayachy L, Ochieng D (2014) Perinatal management of spina bifida. S Afr Med J 104(3):219–221
Morgado T, Figaji A (2014) Hydrocephalus in spina bida. S Afr Med J 104(4):315
Tamburrini G, Frassanito P, Iakovaki K, Pignotti F, Rendeli C, Murolo D, di Rocco C (2013) Myelomeningocele: the management of the associated hydrocephalus. Childs Nerv Syst 29(9):1569–1579
Radmanesh F, Nejat F, Khashab ME, Ghodsi SM, Ardebili HE (2009) Shunt complications in children with myelomeningocele: effect of timing of shunt placement. J Neurosurg Pediatr 3(6):516–520
Northrup H, Volcik KA (2000) Spina bifida and other neural tube defects. Curr Probl Pediatr 30(10):317–332
Rodrigues ABD, Krebs VLJ, Matushita H, de Carvalho WB (2016) Short-term prognostic factors in myelomeningocele patients. Childs Nerv Syst 32(4):675–680
McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ (2003) Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis 36(7):858–862
Caldarelli M, Di Rocco C, La Marca F (1996) Shunt complications in the first postoperative year in children with meningomyelocele. Childs Nerv Syst 12(12):748–754
Khan F, Shamim MS, Rehman A, Bari ME (2013) Analysis of factors affecting ventriculoperitoneal shunt survival in pediatric patients. Childs Nerv Syst 29(5):791–802
Khan F, Rehman A, Shamim MS, Bari ME (2015) Factors affecting ventriculoperitoneal shunt survival in adult patients. Surg Neurol Int 6:25
Öktem I, Menkü A, Özdemir A (2008) When should ventriculoperitoneal shunt placement be performed in cases with myelomeningocele and hydrocephalus? Turk Neurosurg 18(4):387–391
Health Do (2001) Policy guidelines for the management and prevention of genetic disorders. Birth Defects and Disabilities, Department of Health Pretoria
Adzick NS (2010) Fetal myelomeningocele: natural history, pathophysiology, and in-utero intervention. Seminars in fetal and neonatal medicine; 2010. Elsevier, Amsterdam, pp 9–14
De Wals P, Tairou F, Van Allen MI et al (2007) Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med 357(2):135–142
Rabiu T, Tiamiyu L, Awoyinka B (2012) Awareness of spina bifida and periconceptional use of folic acid among pregnant women in a developing economy. Childs Nerv Syst 28(12):2115–2119
Rabiu TB, Adeleye AO (2013) Prevention of myelomeningocele: African perspectives. Childs Nerv Syst 29(9):1533–1540
Africa PoS. Choice on Termination of Pregnancy Act.; 1996
Mweshi M, Amosun S, Ngoma M, Nkandu E (2011) Managing children with spina bifida in sub-Saharan Africa: the Zambian experience? Med J Zambia 38(1):13–23
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mnguni, M.N., Enicker, B.C. & Madiba, T.E. A perspective in the management of myelomeningocoele in the KwaZulu-Natal Province of South Africa. Childs Nerv Syst 36, 1521–1527 (2020). https://doi.org/10.1007/s00381-020-04506-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04506-9