Abstract
Purpose
The most severe form of profound asphyxia in neonates is now known as “total brain injury,” which forms part of the clinical spectrum of hypoxic-ischemic encephalopathy (HIE). Although the magnetic resonance (MR) imaging features of total brain injury remain to be determined, a widespread hyperintensity of the supratentorial brain, known as the “white cerebrum sign,” has been reported in diffusion-weighted images (DWI).
Methods
We examined four neonates who developed severe profound asphyxia.
Results
In the first week of life, all neonates showed the white cerebrum sign on DWI. A follow-up of these cases over a period of 1 month revealed diffuse bilateral multicystic encephalomalacia (MCE) as well as shrinkage of the basal ganglia and thalami (BG/T). These MR findings were common to all neonates, and all the neonates had severe adverse clinical outcomes.
Conclusion
Neonates, who exhibit the white cerebrum sign on MR imaging due to profound asphyxia, develop major disabilities, and MCE with shrinkage of the BG/T suggests miserable outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A lack of blood and oxygen supply to the brains of neonates can result in brain injury termed hypoxic-ischemic encephalopathy (HIE). HIE can be caused by two different patterns of asphyxia: partial or profound [1, 2]. The resulting hypoxia-induced brain damage revealed by magnetic resonance (MR) imaging can be classified into two major categories. The first is a peripheral pattern caused by prolonged partial asphyxia, and the second is the basal ganglia and thalami (BG/T) injury pattern that is caused by transient profound asphyxia. More recently, a new category known as “total brain injury” has been reported [3]. This represents the most severe form of profound asphyxia. This severe form of injury revealed by magnetic resonance imaging (MRI) is considered to be a consequence of prolonged as well as profound asphyxia and exhibits a “white cerebrum sign” on diffusion-weighted images (DWI) [4]. Such cases of severe brain injury in infants have been rarely reported in literature, and thus, the MRI characteristics during the natural progression of total brain injury remain unclear. Our aim in the present study was to clarify the clinical outcomes and the latter MRI features of four infants with total brain injury.
Methods
This study was approved by the institutional review board of the Japanese Red Cross Kyoto Daiichi Hospital(JRCKDH). Oral informed consent was obtained from the parents or guardians of the neonates.
Subjects
Between January 2005 and January 2016, 2068 neonates were treated at the neonatal intensive care unit of JRCKDH. Among them, 59 underwent MRI examination within 1 week of birth for suspected HIE, and 53 of them were clinically diagnosed with HIE. MR images revealed the peripheral pattern injury in 13 neonates, BG/T pattern in 10 neonates, and the white cerebrum pattern in 4 neonates. The remaining 26 patients did not exhibit any remarkable signs on MR images.
Four neonates with the white cerebrum pattern are the subjects of this report. The white cerebrum sign is characterized by widespread supratentorial high-signal intensity on DWI. Two radiologists who had 30 years and 3 years of experience, respectively, in the field of pediatric neuroradiology, identified these four neonates with total brain injury on a consensus basis. The evolution of the clinical and imaging features in these cases was retrospectively reviewed.
Apgar score
The Apgar score was used to assess neonates 1 minute and 5 minutes after birth [5]. Five minutes after birth, scores above 7 are considered to be good. Low Apgar scores have been correlated with increased mortality and are suggested to increase the risk of cerebral palsy.
Clinical staging of HIE and treatments
HIE staging was based on clinical examination using the modified Sarnat staging system [6]. This system assesses muscle tone and tendon reflexes, consciousness levels, and seizures and categorizes the neonates into three grades: mild (I), moderate (II), and severe (III). Based on this staging system and following the CoSTR guidelines for neonatal resuscitation [7], all four neonates in this study underwent treatment for hypothermia.
MR imaging
All neonates underwent a second examination 1 to 2 weeks after birth and a third examination approximately 1 month or more after birth. We used an eight-channel head coil on 1.5-T scanners (Ingenia or Achieva; Philips Medical Systems, Best, the Netherlands). All four patients underwent routine clinical pulse sequences, including spin-echo T1-weighted imaging, fast spin-echo T2-weighted imaging, T2*-weighted imaging, and diffusion-weighted (DW) imaging. The apparent diffusion coefficient (ADC) was measured at the bilateral basal ganglia and thalami, and average values were calculated as described [8].
Clinical outcomes
The outcomes were judged using the Gross Motor Function Classification System (GMFCS) as described previously [9]. This system utilizes a five-level classification system that assesses functional mobility with level V indicating the greatest impairment. While the grading system in infants below the age of two is not well defined, level V indicates a lack of ability to maintain antigravity head and body posture. In addition, their medical charts were reviewed to confirm if they had a diagnosis of epilepsy or had other clinical symptoms.
Results
Clinical course (Table 1)
All four neonates were born at term (36–41 weeks) and were females. The mode of delivery involved a suction cup or Cesarean section in all cases. The causes of HIE were variable and included protraction and uterine rupture. The degree of HIE in terms of the Sarnat score was moderate to severe. All four neonates were treated for hypothermia and also underwent further treatment depending on their individual conditions. Two underwent tracheostomy, one required ventilator support, two required continuous oropharyngeal suction, and three required tube feeding. All four neonates could not perform any purposeful action by themselves and required full assistance for their daily life activities in the chronic phase. This was equivalent to a GMSCF score of V.
MRI findings (Table 2, Figs. 1 and 2)
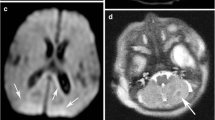
All four neonates demonstrated widespread hyperintensity of the supratentorial brain on initial DWI performed within the first week of life. This was in marked contrast to the almost normal appearance of the cerebellum, except for some hemorrhage or limited high-signal intensity in the dentate nuclei. This combination of features is known as the white cerebrum sign. The ADC for the deep gray matter post asphyxia was decreased to 0.47 × 10−3 ± 0.046 mm2/s (mean ± SD), suggestive of severe injury. DWI showed superior hyperintensity in the cortex as well as deep gray matter within the first week of life. In the second week of life, the deep white matter displayed increased hyperintensity.
MRI findings in case 2. DWI during the first phase (3 days after birth) shows widespread and symmetric hyperintensity in the cerebrum compared with a normal signal in the cerebellum, except in the dentate nucleus, vermis, and tegmentum of the midbrain, and decreased ADC (a–c). Deep white matter does not show higher intensity than the cortex or deep gray matter (a). DWI during the second phase (9 days after birth) shows symmetric hyperintensity in the deep white matter (d) and decreased ADC in the deep white matter (e). T1WI shows hyperintensity in the deep gray matter, while T2WI shows hypointensity. T1WI demonstrates widespread hyperintensity in the cortex of both the dentate nucleus and brainstem, with absence of the posterior limb internal capsule (f, g). During the third phase (34 days after birth), there is widespread cystic change in the cortex and white matter of the cerebrum, with marked shrinkage of the deep gray matter (h, i)
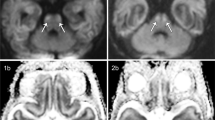
MRI findings in three cases. Case 1 (a–f). DWI during the first phase (1 day after birth) shows asymmetric but widespread hyperintensity in the cerebrum, compared with normal signal in the cerebellum. The cortex and deep gray matter show greater hyperintensity as compared to the deep white matter (a, b). T1WI during the second phase (15 days after birth) shows symmetrical hyperintensity in the deep gray matter and cortex, asymmetrical hypointensity in the subcortex and deep white matter, suggesting cystic change, and absence of the posterior limb internal capsule. Patchy new hemorrhages are evident in the cortex and subcortex of the frontal, temporal, and parietal lobes as clear hyperintense areas (c). T2WI during the second phase shows symmetrical hypointensity in the deep gray matter, and asymmetrical clear hyperintensity in the deep white matter (d). T2*-WI during the second phase shows patchy low intensity signal in the cortex and subcortex of the frontal, temporal, and parietal lobes with no hypointensity in the deep gray matter (e). During the third phase (39 days after birth), there is widespread cystic change in the cortex and white matter of the cerebrum and shrinkage of the deep gray matter (f). Case 3 (g–l). DWI during the first phase (4 days after birth) shows widespread hyperintensities in the whole cerebrum and brainstem with the cortex and deep gray matter showing increased hyperintensity as compared to the deep white matter (g, h). T1WI during the second phase (11 days after birth) reveals hyperintensities in the deep gray matter and cortex, hypointensity in the subcortex and deep gray matter, and absence of the posterior limb sign. There is clear hyperintensity in the trigone of both lateral ventricles (i). T2WI during the second phase shows symmetrical hypointensity in the deep gray matter, and hyperintensity in the subcortex and deep white matter (j). T2*-WI during the second phase indicates low intensity signal in the trigone of both lateral ventricles with no hypointensity in the deep gray matter (k). During the third phase (33 days after birth), there are widespread cystic changes in the cerebrum and shrinkage of the basal ganglia and thalami (l). Case 4 (k–o). DWI during the first phase (2 days after birth) shows widespread hyperintensity in the cerebrum and midbrain with the cortex and deep gray matter showing increased hyperintensity compared to the deep white matter (m, n). T1WI during the second phase (13 days after birth) demonstrates symmetrical hyperintensity in the deep gray matter and cortex with cystic changes clearly evident in the subcortical areas (o). T2WI during the second phase shows symmetrical hypointensity in the deep gray matter and hyperintensity in the subcortex (p). T2*-WI during the second phase shows low intensity in the deep gray matter (q). During the third phase (30 days after birth), there are widespread cystic changes in the cerebrum and shrinkage of the basal ganglia and thalami as well as shrinkage of and hyperintensity in the deep white matter, revealed by T1WI (r)
MRI in the second week of life revealed abnormality of the bilateral BG/T characterized by hyperintensity on T1WI in all four neonates. This hyperintensity in T1WI was suggestive of neuronal necrosis [10] and not hemorrhage. Some subcortical areas showed articulate low intensity on T1WI (with an exception of case 2), suggesting cystic changes. There was hypointensity observed on T2W1 and T2*WI that was variable across the four neonates.
Beyond 1 month of life, MRI strongly suggested multicystic encephalomalacia (MCE) that was associated with shrinkage of the bilateral BG/T.
Discussion
All the neonates showed total brain injury within the first week of life. This phenomenon was first reported as “white cerebrum” by Vermeulen et al. in 2002 [4], and later, Ghei et al. adopted the term “total brain injury” [3]. Total brain injury has been described in only five neonates, and none of them survived. As a consequence, no details of their natural courses have been described [4]. While severe brain injury as seen in this study is known as white brain [11] or cerebral hemispheric devastation [12], the progression patterns of the MRI features of total brain injury remain poorly understood.
DWI in all neonates changed dramatically from the first phase to the second. In the first phase, the white cerebrum sign was apparent and the deep white matter did not show much hyperintensity when compared to the cortex and deep gray matter. In the second phase, the deep gray matter in all neonates showed superior hyperintensity with decreasing ADC. Our findings support one theory of HIE that illustrates rapid brain changes during the early phase of total brain injury [13]. The progression of the early MRI findings of the four neonates emphasizes the fact that the white cerebrum sign does not necessarily indicate uniform hyperintensity in the supratetrial area using DWI.
The present study elucidates the natural course of total brain injury. The four neonates showed MR injury patterns indicative of neuronal necrosis or hemorrhage [10] in the second week of life. Beyond the first month of life, bilateral MCE and shrinkage of the BG/T were evident. These results suggest that the brains of full-term infants who have suffered severely prolonged and profound asphyxia show a white cerebrum pattern followed by MCE and shrinkage of the BG/T.
MCE is a long-term feature that may have various etiologies [14, 15]. Several reports have indicated that it is one of the outcomes of severe and/or prolonged partial asphyxia with an extremely unfavorable prognosis for the hypoxic area [16, 17]. While some studies have considered MCE as an additional category of hypoxic-ischemic brain injury [10], our study revealed a total brain injury pattern more severe than the typical MCE. Interestingly, several studies have reported that MCE and BG/T neuronal necrosis can coexist in neonates with severe asphyxia [18, 19]. Our MRI findings at the different stages demonstrate a white cerebrum injury pattern in the early phase followed by MCE and shrinkage of the BG/T in the latter phase, thus providing additional support to these findings.
Apart from the characteristic MRI findings in these neonates with total brain injury, the clinical outcomes were all unfavorable. While all the four neonates survived to date, they had a very poor prognosis upon discharge. One of them showed mental retardation and epilepsy by the age of four.
This study has a few limitations. The sample size is small, and the period of observation is relatively short for effective evaluation of outcomes. However, this study provides valuable information on the evolution of MRI features in total brain injury.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, Glidden DV, Deming D, Partridge JC, Wu YW, Ashwal S, Ferriero DM (2005) Patterns of brain injury in term neonatal encephalopathy. J Pediatr 146:453–460. https://doi.org/10.1016/j.jpeds.2004.12.026
Zimmerman RA, Bilaniuk LK (2006) Neuroimaging evaluation of cerebral palsy. Clin Perinatol 33:517–544. https://doi.org/10.1016/j.clp.2006.03.005
Ghei SK, Zan E, Nathan JE, Choudhri A, Tekes A, Huisman TA, Izbudak I (2014) MR imaging of hypoxic-ischemic injury in term neonates: pearls and pitfalls. Radiographics 34:1047–1061. https://doi.org/10.1148/rg.344130080
Vermeulen RJ, Fetter WP, Hendrikx L, Van Schie PE, van der Knaap MS, Barkhof F (2003) Diffusion-weighted MRI in severe neonatal hypoxic ischaemia: the white cerebrum. Neuropediatrics 34:72–76. https://doi.org/10.1055/s-2003-39599
Simon LV, Bragg BN APGAR score. Treasure Island (FL): Stat Pearls Publishing; 2018. http://www.ncbi.nlm.nih.gov/books/NBK470569. Accessed 15 June 2019
El-Gamasy MA, Alarabawy R (2018) Relation of serum creatinine to Sarnat scoring and brain computerized tomography of neonates with hypoxic ischemic encephalopathy. A single-center experience. J Pediatr Neurosci 13(4):437–442. https://doi.org/10.4103/JPN.JPN_64_18
International Liaison Committee on Resuscitation (2005) International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Part 7: neonatal resuscitation. Resuscitation. 67(2–3):293–303. https://doi.org/10.1016/j.resuscitation.2005.09.014
Goergen SK, Ang H, Wong F, Carse EA, Charlton M, Evans R, Whiteley G, Clark J, Shipp D, Jolley D, Paul E, Cheong JL (2014) Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: interobserver agreement and MRI predictors of outcome at 2 years. Clin Radiol 69(1):72–81. https://doi.org/10.1016/j.crad.2013.09.001
Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D (2006) Stability of the gross motor function classification system. Dev Med Child Neurol 48(6):424–428. https://doi.org/10.1017/S0012162206000934
Cabaj A, Bekiesińska-Figatowska M, Mądzik J (2012) MRI patterns of hypoxic-ischemic brain injury in preterm and full-term infants - classical and less common MR findings. Pol J Radiol 77(3):71–76
de Vries LS, Groenendaal F (2010) Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiol 52(6):555–566. https://doi.org/10.1007/s00234-010-0674-9
Shankaran S, McDonald SA, Laptook AR, Hintz SR, Barnes PD, Das A, Pappas A, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (2015) Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 167(5):987–993.e3. https://doi.org/10.1016/j.jpeds.2015.08.013
Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SE, Mukherjee P, Glenn OA, Xu D, Partridge JC, Ferriero DM, Vigneron DB (2006) MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol 27(3):533–547
Wolf A, Cowen D (1955) The cerebral atrophies and encephalomalacias of infancy and childhood. Res Publ Assoc Res Nerv Ment Dis 34:199–330
Frigieri G, Guidi B, Costa Zaccarelli S, Rossi C, Muratori G, Ferrari F, Cavazzuti GB (1996) Multicystic encephalomalacia in term infants. Childs Nerv Syst 12:759–764
Krägeloh-Mann I (2004) Imaging of early brain injury and cortical plasticity. Exp Neurol 190:84–90. https://doi.org/10.1016/j.expneurol.2004.05.037
Tekgul H, Serdaroglu G, Yalman O, Tutuncuoglu S (2004) Prognostic correlative values of the late-infancy MRI pattern in term infants with perinatal asphyxia. Pediatr Neurol 31:35–41. https://doi.org/10.1016/j.pediatrneurol.2003.11.012
Garten L, Hueseman D, Stoltenburg-Didinger G, Felderhoff-Mueser U, Weizsaecker K, Scheer I, Boltshauser E, Obladen M (2007) Progressive multicystic encephalopathy: is there more than hypoxia-ischemia? J Child Neurol 22:645–649. https://doi.org/10.1177/0883073807302618
Natsume J, Watanabe K, Kuno K, Hayakawa F, Hashizume Y (1995) Clinical, neurophysiologic, and neuropathological features of an infant with brain damage of total asphyxia type (Myers). Pediatr Neurol 13:61–64
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Editorial support in the form of medical writing was provided by Editage (www.editage.com), a division of Cactus Communications Pvt., Ltd.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Sachiko Koshino, Katsumi Hayakawa, and Koichi Tanda. The first draft of the manuscript was written by Sachiko Koshino, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (the Japanese Red Cross Kyoto Daiichi Hospital(JRCKDH)) and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Oral informed consent was obtained from the parents or guardians of the neonates.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koshino, S., Hayakawa, K., Tanda, K. et al. Magnetic resonance imaging features of four neonates with total brain injury. Childs Nerv Syst 36, 1223–1229 (2020). https://doi.org/10.1007/s00381-019-04457-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04457-w