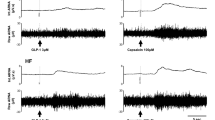
Abstract
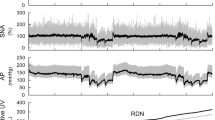
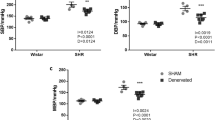
We examined urine excretion during primary acute sympathetic activation (PASA) in Wistar–Kyoto rats with myocardial infarction (MI). The rats underwent unilateral renal denervation (RDN) 7 weeks after coronary artery ligation. 4–10 days later, an acute experiment was performed under anesthetized conditions (n = 8 rats). Isolated carotid sinus pressure was changed stepwise from 60 to 180 mmHg, and the relationship between the arterial pressure (AP) and the normalized urine flow (nUF, urine flow normalized by the body weight) was examined. After obtaining the control data, an angiotensin II type 1 receptor blocker telmisartan (2.5 mg/kg) was intravenously administered. The effects of RDN, telmisartan, and heart weight (biventricular weight) on the relationship between AP and nUF were examined using multiple regression analyses. Regarding the slope of nUF versus AP (nUFslope), the constant term of the regression was positive (0.315 ± 0.069 μL·min−1·kg−1·mmHg−1), indicating that nUF increased with AP. The heart weight had a negative effect on nUFslope (P < 0.05), suggesting that the severity of MI was associated with the impairment of urine excretion. Telmisartan increased nUFslope by 0.358 ± 0.080 μL·min−1·kg−1·mmHg−1 (P < 0.001), whereas RDN had no significant effect on this parameter. The results indicate that unilateral RDN was unable to abolish the effect of the renin–angiotensin system on urine excretion during PASA. Circulating or locally produced angiotensin II, rather than ongoing renal sympathetic nerve activity, played a dominant role in the impairment of urine excretion during PASA in rats with chronic MI.



Similar content being viewed by others
References
Jackson EK (2004) Autonomic control of the kidney. In: Robertson D (ed) Primer on the autonomic nervous system, 2nd edn. Elsevier Academic Press, San Diego, pp 157–161
Kawada T, Hayama Y, Nishikawa T, Suehara S, Sawada S, Tanaka T, Uenohara M, Sugimachi M (2020) Open-loop analysis on sympathetically mediated arterial pressure and urine output responses in rats: effect of renal denervation. J Physiol Sci 70:32
Kawada T, Nishikawa T, Suehara S, Sawada S, Tanaka T, Uenohara M, Yamamoto H, Sugimachi M (2021) Open-loop analysis on sympathetically mediated arterial pressure and urine output responses in spontaneously hypertensive rats: effect of renal denervation. J Physiol Sci 71:13
Rodionova K, Hindermann M, Hilgers K, Ott C, Schmieder RE, Schiffer M, Amann K, Veelken R, Ditting T (2021) AT II receptor blockade and renal denervation: different interventions with comparable renal effects? Kidney Blood Press Res 46:331–341
Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K (2004) Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109:120–124
Shoukas AA, Callahan CA, Lash JM, Haase EB (1991) New technique to completely isolate carotid sinus baroreceptor regions in rats. Am J Physiol 260:H300–H303
Sato T, Kawada T, Miyano H, Shishido T, Inagaki M, Yoshimura R, Tatewaki T, Sugimachi M, Alexander J Jr, Sunagawa K (1999) New simple methods for isolating baroreceptor regions of carotid sinus and aortic depressor nerves in rats. Am J Physiol 276:H326–H332
Heinemann A, Sattler V, Jocic M, Wienen W, Holzer P (1999) Effect of angiotensin II and telmisartan, an angiotensin1 receptor antagonist, on rat gastric mucosal blood flow. Aliment Pharmacol Ther 13:347–355
Balt JC, Mathy MJ, Pfaffendorf M, van Zwieten PA (2001) Inhibition of angiotensin II-induced facilitation of sympathetic neurotransmission in the pithed rat: a comparison between losartan, irbesartan, telmisartan and captopril. J Hypertens 19:465–473
Kent BB, Drane JW, Blumenstein B, Manning JW (1972) A mathematical model to assess changes in baroreceptor reflex. Cardiology 57:295–310
Mohrman D, Heller L (2010) Cardiovascular physiology, 7th edn. McGraw-Hill, New York, pp 246–250
Sato T, Kawada T, Inagaki M, Shishido T, Takaki H, Sugimachi M, Sunagawa K (1999) New analytic framework for understanding sympathetic baroreflex control of arterial pressure. Am J Physiol 276:H2251–H2261
Kawada T, Sugimachi M (2016) Open-loop static and dynamic characteristics of the arterial baroreflex system in rabbits and rats. J Physiol Sci 66:15–41
Glantz SA (2002) Primer of biostatistics, 5th edn. McGraw-Hill, New York, pp 343–362
Glantz SA, Slinker BK, Neilands TB (2016) Primer of applied regression & analysis of variance, 3rd edn. McGraw-Hill, New York, pp 447–452
Krayacich J, Kline RL, Mercer PF (1987) Supersensitivity to NE alters renal function of chronically denervated rat kidneys. Am J Physiol 252:F856–F864
Kawada T, Akiyama T, Li M, Zheng C, Turner MJ, Shirai M, Sugimachi M (2016) Acute arterial baroreflex-mediated changes in plasma catecholamine concentrations in a chronic rat model of myocardial infarction. Physiol Rep 4:e12880
Lohmeier TE, Reinhart GA, Mizelle HL, Han M, Dean MM (1998) Renal denervation supersensitivity revisited. Am J Physiol 275:R1239-1246
Jose PA, Eisner GM, Drago J, Carey RM, Felder RA (1996) Dopamine receptor signaling defects in spontaneous hypertension. Am J Hypertens 9:400–405
Zheng H, Liu X, Rao US, Patel KP (2011) Increased renal ENaC subunits and sodium retention in rats with chronic heart failure. Am J Physiol Renal Physiol 300:F641–F649
Klabunde RE (2012) Cardiovascular physiology concepts, 2nd edn. Lippincott Williams & Wilkins, Philadelphia, pp 171–173
DiBona GF, Kopp UC (1997) Neural control of renal function. Physiol Rev 77:75–197
Davis JO, Freeman RH (1976) Mechanisms regulating renin release. Physiol Rev 56:1–56
Stein JH (1990) Regulation of the renal circulation. Kidney Int 38:571–576
Clase CM, Barzilay J, GaoP SA, Schmieder RE, Tobe S, Teo KK, Yusuf S, Mann JFE (2017) Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int 91:683–690
Zheng H, Liu X, Katsurada K, Patel KP (2019) Renal denervation improves sodium excretion in rats with chronic heart failure: effects on expression of renal ENaC and AQP2. Am J Physiol Heart Circ Physiol 317:H958–H968
Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA (2003) Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41:1143–1150
Saleh MG, Sharp SK, Alhamud A, Spottiswoode BS, van der Kouwe AJW, Dvies NH, Franz T, Meintjes EM (2012) Long-term left ventrulcar remodeling in rat model of nonreperfused myocardial infarction: sequential MR imaging using a 3T clinical scanner. J Biomed Biotechnol 2012:504037
Schrier RW, Tomas B, Harbottle JA (1972) Mechanism of the antidiuretic effect associated with interruption of parasympathetic pathways. J Clin Invest 51:2613–2620
Kishi T, Hirooka Y, Sunagawa K (2014) Telmisartan reduces mortality and left ventricular hypertrophy with sympathoinhibition in rats with hypertension and heart failure. Am J Hypertens 27:260–267
Sueta D, Koibuchi N, Hasegawa Y, Toyama K, Uekawa K, Katayama T, Ma M, Nakagawa T, Ogawa H, Kim-Mitsuyama S (2014) Telmisartan exerts sustained blood pressure control and reduces blood pressure variability in metabolic syndrome by inhibiting sympathetic activity. Am J Hypertens 27:1464–1471
Khan AH, Imig JD (2011) Telmisartan provides better renal protection than valsartan in a rat model of metabolic syndrome. Am J Hypertes 24:816–821
Acknowledgements
This study was partly supported by grants from Grants-in-Aid for Scientific Research (JSPS KAKENHI 20K20622), Salt Science Research Foundation (2029, 2125), and Takeda Medical Research Foundation. Part of this work was carried out by a research grant from NTT research, Inc. The authors confirm that these parties had no influence in the study design, contents of the article, or selection of this journal.
Author information
Authors and Affiliations
Contributions
TK: conceived and designed the study. TK and ML: performed the experiments. TK: analyzed data. TK, ML, SSu, SSa, CZ, KU, MS, and KS: interpreted the results of the experiments. TK: prepared the figures and drafted the manuscript. TK: edited and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
Japan Society for the Promotion of Science, 20K20622, Toru Kawada,Salt Science Research Foundation, 2029, 2125, Toru Kawada, Takeda Medical Research Foundation, Toru Kawada.
Conflict of interest
The authors declare no conflict of interest regarding this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
The line representing the relationship between AP and nUF in each condition was drawn based on the following equation (Eq. A1).
where nUFintercept and nUFslope were determined using values listed in Table 3. For the control data on the intact side, the mean values of C were adopted, and the relationship between AP and nUF was described by the following equation (Eq. A2).
For the control data on the denervated side, the mean values of BRDN were considered, and the relationship between AP and nUF was obtained by the following equation (Eq. A3).
For the data after the administration of telmisartan on the intact side, the mean values of BTLM were considered, and the relationship between AP and nUF was obtained by the following equation (Eq. A4).
For the data after the administration of telmisartan on the denervated side, the mean values of BRDN and BTLM were considered, and the relationship between AP and nUF was obtained by the following equation (Eq. A5).
Rights and permissions
About this article
Cite this article
Kawada, T., Li, M., Suehara, S. et al. Angiotensin II inhibition increases diuresis during acute sympathetic activation in intact and denervated kidneys in rats with chronic myocardial infarction. Heart Vessels 37, 1636–1646 (2022). https://doi.org/10.1007/s00380-022-02110-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-022-02110-2