Abstract
Myocardial perfusion imaging (MPI) using Single Photon Emission Computed Tomography has been established as a standard noninvasive tool for risk stratification of coronary artery disease (CAD). We evaluated the diagnostic performance of on-site workstation-based computed tomography-derived fractional flow reserve (CT-FFR) in comparison with MPI using invasive fractional flow reserve (invasive FFR) as a gold standard. We enrolled 97 patients with suspected CAD. Diagnostic performance of CT angiography (CTA), and CT-FFR was compared in 105 lesions of 97 patients. Invasive FFR ≤ 0.8 was detected in 38 (36%) lesions. Diagnostic performance of CT-FFR was improved compared with CTA (AUC 0.83 vs. 0.60, p < 0.0001). The lesions with both CTA and MPI findings (n = 47), invasive FFR ≤ 0.8 was detected in 19 (40.4) lesions. CT-FFR (AUC 0.81, 95% CI 0.72–0.94) significantly improved diagnostic performance compared with CTA-50% (AUC 0.59, p = 0.00019) and MPI (AUC 0.64, p = 0.0082). In lesions with ≥ 50% on CTA (n = 42), diagnostic accuracy of CT-FFR (AUC 0.81) was significantly superior to MPI (AUC 0.64, p = 0.0239). In conclusions, CT-FFR improved diagnostic accuracy to detect invasive FFR ≤ 0.8 compared with luminal stenosis on CTA and ischemia on MPI. Patients with ≥ 50% stenosis on CTA would be the candidates for CT-FFR.
Similar content being viewed by others
Introduction
Since the development of coronary computed tomography angiography (CTA), the management of coronary artery disease (CAD) using noninvasive imaging has been changing. Myocardial perfusion imaging (MPI) using Single Photon Emission Computed Tomography (SPECT) has been used for risk stratification of CAD with established evidence in the last 20 years [1, 2]. In the last decade, CTA was widely used to rule out obstructive stenosis [3, 4]. Several randomized control studies have demonstrated the superiority of CTA over functional tests, resulting in reductions in the rates of invasive coronary angiography (ICA) or major events [5,6,7,8,9].
Currently, invasive fractional flow reserve (FFR), not ICA, has become the gold standard to select patients requiring revascularization [10,11,12,13]. Noninvasive assessment of CT-derived FFR was developed recently. The clinical usefulness of CT-derived FFR has been reported previously [14,15,16,17,18,19]. Most assessments of CT-derived FFR have consisted of comparisons between CT and invasive FFR. In this study, we evaluated the diagnostic performance of on-site workstation-based CT-FFR in comparison with MPI using invasive FFR as the gold standard.
Materials and methods
Study population
The patients were retrospectively enrolled. In patients undergoing invasive FFR from May 2016 to December 2018, patients with CTA within 3 months were screened. Patients aged < 20 years, after coronary artery bypass grafting, or with atrial fibrillation were excluded from the study. In patients who underwent CTA, only those eligible for CT-FFR analysis were included in this study; images were scanned with 320-slice CT over 70–99% of R–R interval in one heartbeat. In patients with invasive FFR and CTA, the presence of MPI data was also screened. Previously stented vessels, lesions with previous myocardial infarction (MI), and total occluded lesions were excluded from the analysis; non-stented lesions of patients with previous percutaneous coronary intervention (PCI) were included for analysis. Information of previous MI was obtained from self-reported medical history or past medical history on the chart. Attending physicians decided patient management including selection of CTA, MPI, ICA, and invasive FFR as a daily clinical practice, not for research. CT-FFR value was not provided to the physicians clinically. We evaluated the diagnostic performance of on-site workstation-based CT-FFR in comparison with CTA and MPI using invasive FFR as the gold standard. The institutional ethics committee at Fujita Health University approved the study.
CTA data acquisition
All patients underwent prospective ECG-gated CT scan with data acquisition over 70–99% of the R–R interval in one heartbeat using 320-slice CT (Aquilion One Vision; Canon Medical Systems Corp., Otawara, Japan). Coronary artery calcium score was performed by the following parameters: 120 kV, 150 mA, and 3 mm thickness to calculate Agatston score [20]. Calcification at the stented lesion was excluded from the analysis. 320-slice CT was performed with a collimation of 320 × 0.5 mm and rotation time of 275 or 350 ms. Tube voltage was 120 kV, and maximal tube current was 300–580 mA depending on body weight. For the contrast-enhanced scan, 245 mgI/kg of contrast medium was injected in 12 s followed by 20 ml saline at 3.0 ml/s. All patients received sublingual nitroglycerine. Before imaging, in patients without contraindications, oral metoprolol and/or intravenous landiolol was administered if the pre-scan heart rate was > 65 bpm. In patients with HR ≤ 65 bpm at scanning, axial scan was performed with prospective gated scan at 70–99% of R–R interval in one heart beat. Patients with HR 65 > were scanned at more than 2 heart beats. Patients who were scanned at systole or more than 2 beats at diastole were not included in this study, because these data were not eligible for CT-FFR analysis. The raw data of the CT scans were reconstructed using retrospective ECG-gated half reconstruction. Median (interquartile range: IQR) radiation exposure was 2.9 (2.1–4.0) mSv. The reconstructed image data of CT were transferred to an on-site computer workstation for post-processing: an optimal single phase data to ZIOSTATION System 1000 (Amin/ZIO, Japan) for evaluation of luminal stenosis and 4 volume data sets at 70, 80, 90, and 99% of R–R interval to sure-Cardio CT-FFR (Research Version, W.I.P., Canon Medical Systems, Japan) for CT-FFR analysis.
Luminal stenosis on CTA
Two cardiologists who were unaware of the patient’s clinical information, CT-FFR, MPI, and invasive FFR findings interpreted the luminal stenosis on CTA images by consensus based on visual estimation. Coronary arteries were divided into 15 segments based on the American Heart Association (AHA) classification, and the stenosis grade was evaluated in coronary arteries with a diameter of ≥ 2 mm. Stenosis was visually graded from 0 to 5 [0: absence of plaque and no luminal stenosis, 1: < 25% stenosis, 2:25–49% stenosis, 3:50–69% stenosis, 4:70–99% stenosis, 5 occluded] [21]. For stenosis grading analyses, lesions with ≥ 50% was defined as obstructive. For purposes of classification for per-vessel analyses, we considered 4 arterial territories: left main artery, left anterior descending artery (LAD), left circumflex artery (LCx), and right coronary artery (RCA). Obstructive CAD in the diagonal branches, obtuse marginal branches, and posterolateral branches was considered as part of the LAD, LCx, and RCA system, respectively. The posterior descending artery was considered as part of the RCA or LCx system, depending on the coronary artery dominance.
CT-FFR calculation
CT-FFR analysis was performed using a dedicated algorithm [22, 23] employing fluid structure interaction for patient-specific CT-FFR calculation. The CT-FFR analysis was performed by 2 cardiologists blinded to the results of the invasive FFR and MPI. Four CT images in one patient were reconstructed from the available phases (at 70%, 80%, 90%, and 99% of R–R interval). Semi-automatic calculation of CT-FFR was performed on a workstation as previously reported. CT-FFR values could be provided at any point from ostium of coronary artery to the distal with vessel diameter of 1.8 mm. Totally occluded vessels were not eligible for CT-FFR analysis. Based on the previous study, CT-FFR values were obtained at 2 cm distal to the distal end of the stenotic lesion [24, 25]. Inter- and intra-observer variabilities of CT-FFR were expressed as kappa.
Invasive coronary angiography and FFR
ICA was performed according to standard practice. Pressure measurements were performed using a 0.014-inch pressure guide wire (FFR; PressureWire Certus, St. Jude Medical, St. Paul, Minnesota) [11]. The pressure wire was calibrated and equalized with the aortic pressure before being placed distal to the stenosis and in the distal third of the coronary artery being interrogated. Invasive FFR was performed in at least one vessel of diameter ≥ 2 mm and visual stenosis between 10% and 90% chosen at the discretion of the operator, blinded to the CT findings. FFR was recorded in the distal coronary artery following intracoronary nitric acid (2 mg) and steady-state hyperaemia achieved with administration of intravenous adenosine at 140 lg/kg/min. The pressure sensor was then pulled back into the tip of the guiding catheter and only runs with ≤ 0.05 drift were accepted for analysis. FFR was measured as the mean distal coronary pressure (Pd) divided by the mean aortic pressure (Pa) during maximal hyperemia.
A FFR value of ≤ 0.8 was chosen to define hemodynamically significant ischemia.
Adenosine stress myocardial perfusion imaging
Adenosine stress thallium-201 (201Tl) or 99mTechnetium-tetrofosmin (Tf) SPECT was performed. Adenosine was administered intravenously at 0.12 mg/kg/min for 6 min. 201Tl and Tf were injected during adenosine infusion, and Tf was also injected before the delayed image was taken. Early and delayed images were acquired 15 min and 3 h after tracer injection, respectively. Myocardial SPECT imaging was performed using a dual-detector gamma camera (SymbiaT6/T16, Siemens AG, Munich, Germany). Delayed images were obtained with 17 views per head. All SPECT images were obtained with the same method. The SPECT images were divided into 17 standardized myocardial segments as defined by the AHA. Distribution was evaluated visually by 5-point scoring in stress (summed stress score; SSS) and delayed images (summed rest score; SRS): 0 = normal, 1 = slight reduction of tracer uptake, 2 = moderate reduction of uptake, and 4 = absence of uptake. Summed difference score (SDS) was obtained as followed: SDS = SSS–SRS. SDS ≥ 2 was defined as abnormal MPI images. In 17-segment models, regions were divided into RCA, LAD, and LCx based on the guidelines [26, 27]. Images were analyzed independently by 2 cardiologists who were unaware of the CT and invasive FFR data.
Statistical analysis
Statistical analyses were performed with the JMP software (version 12.0, SAS Institute, Cary, North Carolina), and p values of < 0.05 were considered statistically significant. Shapiro–Wilk test was used to assess the normality of continuous data. All continuous measurements are expressed as mean ± standard deviation (SD) for normally distributed variables or median and IQR (25th percentile–75th percentile) for nonparametric data. Categorical variables are presented as frequencies (percentages). ICC and Bland–Altman analysis were calculated to evaluate the relation between CT-FFR and invasive FFR. Diagnostic accuracy of CT findings and MPI to detect ischemia were presented as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Receiver-operating characteristic (ROC) area under the curve (AUC) analysis was undertaken to evaluate the discriminatory ability of coronary CTA, CT-FFR, and MPI to detect invasive FFR ≤ 0.8. AUCs were also compared by the DeLong method [28].
Results
Patient characteristics
In patients who underwent CTA using 320-slice CT (n = 1996), 1523 (76%) patents were eligible for CT-FFR analysis. Of 1523 patients, 97 who had invasive FFR were included in this study (mean age 70 ± 9 years, 75% male); MPI was also available in 47 vessel-territories of 43 patients (Table 1). The reasons for the diagnostic test included typical chest pain (n = 47), atypical chest symptoms (n = 23), abnormal electrocardiography without chest symptoms (n = 21), assessment of non-culprit lesion of acute coronary syndrome (n = 3), arrythmia (n = 2), and congestive heart failure (n = 1). Nineteen (20%) patients had a history of MI, and 22 (23%) a history of PCI (stent implantation: 1 vessel = 18 and 2 vessels = 2). Average Agatston score on CTA was 601 ± 821.
The reasons why patients underwent both CTA and MPI were as follows: MPI followed by abnormal CTA (n = 33), CTA followed by abnormal MPI (n = 4), MPI followed by abnormal CTA and borderline ICA (n = 3), and CTA for plaque assessment after MPI and ICA (n = 3).
Diagnostic accuracy of CT findings
In comparison with invasive FFR as the gold standard, the diagnostic accuracy of luminal stenosis on CTA and CT-FFR was evaluated in 105 vessels of 97 patients. Invasive FFR ≤ 0.8 was detected in 38 (36%) vessels. Kappa values for inter- and intra-observer variability of CT-FFR were [0.79 (95% CI 0.53–1, p = 0.0003) and [0.91 (95% CI 0.80–1, p < 0.0001)].
Figure 1 shows the distribution of CT-FFR and invasive FFR in each stenosis grading on CTA.
Distribution of CT-FFR and invasive FFR in each stenosis grading on CTA. All patients with stenosis < 50% on CTA (n = 14, 13%) had CT-FFR > 0.8 (a) and invasive FFR > 0.8 (b). In lesions with > 50% stenosis on CTA (n = 91), CT-FFR ≤ 0.8 was detected in 42 lesions and invasive FFR ≤ 0.8 in 38 lesions
All patients with stenosis < 50% on CTA (n = 14, 13%) had CT-FFR > 0.8 and invasive FFR > 0.8. In lesions with ≥ 50% stenosis on CTA (n = 91), CT-FFR ≤ 0.8 was detected in 42 lesions and invasive FFR ≤ 0.8 in 38 lesions. To detect invasive FFR ≤ 0.8, true positives were 38 (41.8%) and false positives were 53 (58.2%) by CTA (Table 2). CT-FFR showed fewer false positives (n = 11). Diagnostic performance of CT-FFR (AUC 0.83, 95% CI 0.75–0.90) was significantly better as compared with CTA (AUC 0.60, 95% CI 0.56–0.65, p < 0.0001). Diagnostic accuracy in all lesions was 83%; diagnostic accuracy of CT-FFR 0.62–0.82 (n = 27) was 70%. In lesions with CT-FFR ≤ 0.62 (n = 18), true positives were 83%, and in lesions with CT-FFR > 0.82 (n = 60), true negatives were 90%.
CTA, CT-FFR, and MPI
Of 105 lesions, 47 vessel-territories were also evaluated by MPI.
The number of diseased vessels (≥ 50% stenosis on ICA) was as follows: 0 vessel disease (VD) = 18, 1VD = 18, 2VD = 8, and 3VD = 3 (Table 3). The diagnostic performance of CTA, CT-FFR, and MPI did not depend on the number of diseased vessels. In lesions with CT and MPI, abnormal FFR was detected in 19 (40.4%) lesions (Table 4).The diagnostic performance of CT-FFR showed sensitivity 84 and 80%, specificity 78 and 73%, PPV 76 and 80%, NPV 86 and 73%, and diagnostic accuracy 81 and 77%, respectively (Table 4). MPI was superior to CT-FFR in specificity (96 and 91%) and PPV (86 and 86%), but showed low sensitivity (32%) and NPV (63%).
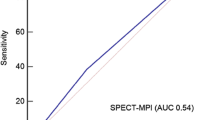
CT-FFR (AUC 0.81, 95% CI 0.72–0.94) significantly improved diagnostic performance in ROC analysis compared with CTA-50% (AUC 0.59, 95% CI 0.52–0.66, p = 0.00019) and MPI (AUC 0.64, 95% CI 0.53–0.75, p = 0.0082) (Fig. 2). In patients with ≥ 50% on CTA, the diagnostic accuracy of CT-FFR (AUC 0.81, 95% CI 0.69–0.93) was significantly superior to MPI (AUC 0.64, 95% CI 0.52–0.75, p = 0.0239) (Table 4). In patients with ≥ 70% stenosis on CTA, diagnostic performance of CT-FFR (AUC 0.76, 95% CI 0.59–0.94) was improved compared with MPI (AUC 0.66, 95% CI 0.50–0.81, p = 0.33), but the difference was not significant.
A representative case is shown in Fig. 3.
Discussion
In this study, we evaluated the diagnostic performance of on-site workstation-based CT-FFR in comparison with MPI using invasive FFR as the gold standard. The salient findings of this study was that CT-FFR showed improved diagnostic accuracy to detect invasive FFR ≤ 0.8 as compared with luminal stenosis on CTA and ischemia on MPI.
Diagnostic performance of on-site CT-FFR
Luminal stenosis on CTA is widely used and has become a gatekeeper for ICA with high sensitivity and NPV for ruling out obstructive stenosis [3, 4]. However, invasive FFR, not ICA, has become the gold standard to select patients requiring revascularization [10,11,12,13]. Noninvasive assessment of off-site CT-derived FFR was proposed by HeartFlow (FFRCT; HeartFlow, Inc, Redwood City, CA). HeartFlow FFRCT is measured using principles of computational fluid dynamics based on coronary CTA images, which permit computation of coronary flow and pressures along the length of the entire coronary tree. The diagnostic accuracy of FFRCT was improved compared with CTA stenosis using invasive FFR as the golden standard, especially regarding (54–82 vs. 25–42%) and PPV (65–85 vs. 40–61%) [14,15,16]. One of the limitations of HeartFlow FFRCT so far is the requirement to outsource the data for analysis and receive the results only at a later time. On the other hand, on-site CT-FFR, which was used in the present study, was newly developed with a different algorithm using fluid structure interaction analysis without adenosine infusion, which allowed on-site analysis, and with the results obtained within about 30–60 min. However, performing CT-FFR analysis simultaneously may affect the workflow of CTA in the hospital. It would be important to establish an optimal workflow of CTA including CT-FFR. For example, training for CT-FFR analysis would be needed. Ri et al. reported that CT-FFR values displayed good reproducibility even when the postprocessing was performed by inexperienced observers given only a brief training for 20 min [29]. Another disadvantage of on-site CT-FFR compared with HeartFlow FFRCT was that on-site CT-FFR was limited to use with one heart beat scanned data by 320-slice CT [17]. In the present study, 76% of patients were eligible for CT-FFR analysis; 24% patients were scanned at more than 2 heart beasts because of high heart rate or arrythmia.
In the previous studies, the diagnostic accuracy of on-site CT-FFR was comparable to that of off-site HearFlow FFTCT; CT-FFR was improved in specificity (79–84%) and PPV (74–76%) compared with CTA stenosis (specificity 43–74%, PPV 30–56%) [17,18,19].
In the present study, the diagnostic accuracy of on-site CT-FFR was improved compared with luminal stenosis on CTA (diagnostic accuracy 83 vs. 51%, specificity 82 vs. 18%, PPV 76 vs. 45%), which was consistent with the previous reports with on-site CT-FFR and off-site HeartFlow FFRCT.
One of the limitations of CTA for assessment of stenosis or ischemia was low PPV. Although CT-derived FFR including both HeartFlow FFRCT and on-site CT-FFR improved PPV compared with CTA stenosis, it is not be perfect. The reasons underlying the low PPV of CT-derived FFR likely include poor image quality and calcification [15, 16, 30]. Recently, development of CT-derived FFR using machine learning has been reported, and the diagnostic performance was improved using machine learning [31,32,33]. Development of CT-derived FFR using machine learning would help improve the diagnostic performance including specificity and PPV.
On-site CT-FFR or MPI
Most of the previous reports focused on CT-derived FFR were compared with CTA stenosis and invasive FFR. In the present study, the diagnostic performance of CT-FFR was compared with MPI using invasive FFR as the gold standard. Functional testing using MPI has been widely used for risk stratification of CAD with established evidence [1, 2]. In the present study, MPI demonstrated low sensitivity and high specificity to detect positive invasive FFR, which was consistent with the results of previous studies; sensitivity of MPI compared with ICA has been reported as 70–90%. Sensitivity of MPI compared to invasive FFR has shown the relatively low range of values of 42–61% [34, 35].
One of the limitations of MPI is the false negative result of a normal study in patients with 3-VD [5, 36, 37]. However, in the present study, the diagnostic performance did not depend on the number of diseased vessels. Furthermore, the overall diagnostic performance of CT-FFR was superior to that of MPI. The discrepancy between invasive FFR and coronary flow reserve (CFR) was attributed previously to differences between luminal stenosis and microvascular dysfunction [5]; MPI represents CFR more than FFR. On the other hand, CT-FFR was developed to serve as an equivalent tool to invasive FFR to detect ischemia. The differences between FFR and MPI could account for the discordant results between CT-FFR and MPI. Thus it was reasonable for CT-FFR to be more closely related with invasive FFR than MPI in the present study.
Diagnostic strategy
Several studies have compared CTA and functional testing for patients with stable chest pain [38, 39]. They demonstrated that CTA could reduce the rate of ICA. Furthermore, in patients undergoing ICA, the percentage of non-obstructive stenosis was decreased in the CTA group. In addition to demonstrating the superiority of CTA over functional testing in identifying obstructive stenosis by ICA, the randomized controlled trials are suggestive of a beneficial effect on prognostic outcomes of CTA vs. functional test guided management [38,39,40,41]. The present study demonstrated the superiority of CT-FFR over CTA and MPI to detect ischemia as compared with invasive FFR.
The National Institute of Health and Care Excellence (NICE) proposes that patients with stable chest pain undergo CTA as the first-line test and noninvasive functional test as the second-line test [41]. The NICE also describes that HeartFlow FFRCT should be considered as an option for patients with stable, recent onset chest pain who are offered coronary CT angiography as part of the NICE chest pain pathway.
In the present study, no patient with stenosis of < 50% on CTA showed positive invasive FFR (≤ 0.8) or positive CT-FFR (≤ 0.8). Furthermore, the diagnostic performance of CT-FFR was superior to that of MPI in patients with ≥ 50% stenosis on CTA. Based on the present results, patients with luminal stenosis ≥ 50% on CTA would be candidates for CT-FFR as a gatekeeper for ICA. It is unclear if it is possible to judge the indication for PCI based on the results of on-site CT-FFR. In the present study, compared to the diagnostic accuracy of all lesions (83%), the diagnostic accuracy of CT-FFR from 0.62 to 0.82 was relatively low (70%). It will be necessary to assess prognosis based on CT-FFR before establishing the clinical implications of decision making by CT-FFR.
Limitations of this study
First, the development of CT-FFR in the present study is a work-in-progress. Second, this is not a randomized control trial. Selection of imaging modalities was depended on the attending physician. Furthermore, this study focused on only a small number of patients. Patient population could be biased. A multicenter study with a larger population would be needed. However, the present study demonstrated real world data, thereby helping to clarify the clinical implications of CT-FFR.
Conclusions
On-site workstation-based CT-FFR using fluid structure interaction analysis was a useful tool to detect ischemia. CT-FFR improved diagnostic accuracy to detect invasive FFR ≤ 0.8 compared with luminal stenosis on CTA and ischemia on MPI.
References
Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA (1998) Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 97(6):535–543
Hachamovitch R, Berman DS, Kiat H, Bairey-Merz N, Cohen I, Cabico JA, Friedman JD, Germano G, Van Train KF, Diamond GA (1995) Gender-related differences in clinical management after exercise nuclear testing. J Am Coll Cardiol 26(6):1457–1464
Paech DC, Weston AR (2011) A systematic review of the clinical effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of suspected coronary artery disease. BMC Cardiovasc Disord 11:32
Hamon M, Morello R, Riddell JW, Hamon M (2007) Coronary arteries: diagnostic performance of 16- versus 64-section spiral CT compared with invasive coronary angiography-meta-analysis. Radiology 245(3):720–731
Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huahg M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL (2015) Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 372(14):1291–1300
McKavanagh P, Lusk L, Ball PA, Verghis RM, Agus AM, Trinick TR, Duly E, Walls GM, Stevenson M, James B, Hamilton A, Harbison MT, Donnelly PM (2015) A comparison of cardiac computerized tomography and exercise stress electrocardiogram test for the investigation of stable chest pain: the clinical results of the CAPP randomized prospective trial. Eur Heart J Cardiovasc Imaging 16(4):441–448
Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC (2018) Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 379(10):924–933
Chang HJ, Lin FY, Gebow D, An HY, Andreini D, Bathina R, Baggiano A, Beltrama V, Cerci R, Choi EY, Choi JH, Choi SY, Chung N, Cole J, Doh JH, Ha SJ, Her AY, Kepka C, Kim JY, Kim JW, Kim SW, Kim W, Pontone G, Valeti U, Villines TC, Lu Y, Kumar A, Cho I, Danad I, Han D, Heo R, Lee SE, Lee JH, Park HB, Sung JM, Leflang D, Zullo J, Shaw LJ, Min JK (2019) Selective referral using CCTA versus direct referral for individuals referred to invasive coronary angiography for suspected CAD: a randomized, controlled, open-label trial. JACC Cardiovasc Imaging 12(7 Pt 2):1303–1312
Pontone G, Andreini D, Bartorelli AL, Bertella E, Cortinovis S, Mushtaq S, Foti C, Annoni A, Formenti A, Baggiano A, Conte E, Bovis F, Veglia F, Ballerini G, Fiorentini C, Agostoni P, Pepi M (2013) A long-term prognostic value of CT angiography and exercise ECG in patients with suspected CAD. JACC Cardiovasc Imaging 6(6):641–650
Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijins W (2001) Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 103(24):2928–2934
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, FAME study investigators (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360(3):213–224
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF, FAME 2 Trial Investigators (2012) Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 367(11):991–1001
Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink JH, Rioufol G, Toth GG, Piroth Z, Witt N, Fröbert O, Kala P, Linke A, Jagic N, Mates M, Mavromatis K, Samady H, Irimpen A, Oldroyd K, Campo G, Rothenbühler M, Jüni P, De Bruyne B, FAME 2 Investigators (2018) Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 379(3):250–259
Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK (2011) Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms: results from the prospective multicenter DISCOVER-FLOW (Diagnosis of ischemia-causing stenoses obtained via noninvasive fractional flow reserve) study. J Am Coll Cardiol 58(19):1989–1997
Min JK, Berman DS, Budoff MJ, Jaffer FA, Leipsic J, Leon MB, Mancini GB, Mauri L, Schwartz RS, Shaw LJ (2011) Diagnostic accuracy of fractional flow reserve from anatomic computed tomographic angiography: the DeFACTO study. J Cardiovasc Comput Tomogr 5(5):301–309
Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park SJ, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S, NXT Trial Study Group (2014) Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease The NXT trial (Analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol 63(12):1145–1155
Ko BS, Cameron JD, Munnur RK, Wong DTL, Fujisawa Y, Sakaguchi T, Hirohata K, Hislop-Jambrich J, Fujimoto S, Takamura K, Crossett M, Leung M, Kuganesan A, Malaiapan Y, Nasis A, Troupis J, Meredith IT, Seneviratne SK (2017) Noninvasive CT-derived FFR based on structural and fluid analysis a comparison with invasive FFR for detection of functionally significant stenosis. JACC Cardiovasc Imaging 10(6):663–673
Fujimoto S, Kawasaki T, Kumamaru KK, Kawaguchi Y, Dohi T, Okonogi T, Ri K, Yamada S, Takamura K, Kato E, Kato Y, Hiki M, Okazaki S, Aoki S, Mitsouras D, Rybicki FJ, Daida H (2019) Diagnostic performance of on-site computed CT-fractional flow reserve based on fluid structure interactions: comparison with invasive fractional flow reserve and instantaneous wave-free ratio. Eur Heart J Cardiovasc Imaging 20(3):343–352
Ihdayhid AR, Sakaguchi T, Linde JJ, Sørgaard MH, Kofoed KF, Fujisawa Y, Hislop-Jambrich J, Nerlekar N, Cameron JD, Munnur RK, Crosset M, Wong DTL, Seneviratne SK, Ko BS (2018) Performance of computed tomography-derived fractional flow reserve using reduced-order modelling and static computed tomography stress myocardial perfusion imaging for detection of haemodynamically significant coronary stenosis. Eur Heart J Cardiovasc Imaging 19(11):1234–1243
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15(4):827–832
Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, Pontone G, Raff GL (2014) SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of cardiovascular computed tomography guidelines committee. J Cardiovasc Comput Tomogr 8(5):342–358
Hirohata K, Kano A, Goryu A, Ooga J, Hongo T, Higashi S, Fujisawa Y, Wakai S, Arakita K, Ikeda Y, Kaminaga S, Ko B, Seneviratne S (2015) A novel CT-FFR method for the coronary artery based on 4D-CT image analysis and structural and fluid analysis. SPIE Med Imaging 9412:941220
Kato M, Hirohata K, Kano A, Higashi S, Goryu A, Hongo T, Kaminaga S, Fujisawa Y (2015) Fast CT-FFR analysis method for the coronary artery based on 4D-CT image analysis and structural and fluid analysis. IMECE 51124:1–10
Kueh H, Mooney J, Ohana M, Kim U, Blanke P, Grover R, Sellers S, Ellis J, Murphy D, Hague C, Bax JJ, Nørgaard BL, Rabbat M, Leipsic JA (2017) Fractional flow reserve derived from coronary computed tomography angiography reclassification rate using value distal to lesion compared to lowest value. J Cardiovasc Comput Tomogr 11(6):462–467
Rabbat MG, Berman DS, Kern M, Raff G, Chinnaiyan K, Koweek L, Shaw LJ, Blanke P, Scherer M, Jensen JM, Lesser J, Nørgaard BL, Pontone G, De Bruyne B, Bax JJ, Leipsic J (2017) Interpreting results of coronary computed tomography angiography-derived fractional flow reserve in clinical practice. J Cardiovasc Comput Tomogr 11(5):383–388
Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, Ficaro EP, Freeman MR, Hendel RC, Jain D, Leonard SM, Nichols KJ, Polk DM, Soman P, American Society of Nuclear Cardiology (2010) Single photon-emission computed tomography. J Nucl Cardiol 17(5):941–973
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American heart association. Circulation 105(4):539–542
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Ri K, Kumamaru KK, Fujimoto S, Kawaguchi Y, Dohi T, Yamada S, Takamura K, Kogure Y, Yamada N, Kato E, Irie R, Takamura T, Suzuki M, Hori M, Aoki S, Daida H (2018) Noninvasive computed tomography-derived fractional flow reserve based on structural and fluid analysis: reproducibility of on-site determination by unexperienced observers. J Comput Assist Tomogr 42(2):256–262
Nørgaard BL, Gaur S, Leipsic J, Ito H, Miyoshi T, Park SJ, Zvaigzne L, Tzemos N, Jensen JM, Hansson N, Ko B, Bezerra H, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S (2015) Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT trial. JACC Cardiovasc Imaging 8(9):1045–1055
Tesche C, Otani K, De Cecco CN, Coenen A, De Geer J, Kruk M, Kim YH, Albrecht MH, Baumann S, Renker M, Bayer RR, Duguay TM, Litwin SE, Varga-Szemes A, Steinberg DH, Yang DH, Kepka C, Persson A, Nieman K, Schoepf UJ (2019) Influence of coronary calcium on diagnostic performance of machine learning CT-FFR: results from MACHINE registry. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2019.06.027
Tesche C, De Cecco CN, Baumann S, Renker M, McLaurin TW, Duguay TM, Bayer RR, Steinberg DH, Grant KL, Canstein C, Schwemmer C, Schoebinger M, Itu LM, Rapaka S, Sharma P, Schoepf UJ (2018) Coronary CT angiography-derived fractional flow reserve: machine learning algorithm versus computational fluid dynamics modeling. Radiology 288(1):64–72
van Rosendael AR, Maliakal G, Kolli KK, Beecy A, Al’Aref SJ, Dwivedi A, Singh G, Panday M, Kumar A, Ma X, Achenbach S, Al-Mallah MH, Andreini D, Bax JJ, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJW, Cury RC, DeLago A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann PA, Kim YJ, Leipsic JA, Maffei E, Marques H, Pontone G, Raff GL, Rubinshtein R, Shaw LJ, Villines TC, Gransar H, Lu Y, Jones EC, Peña JM, Lin FY, Min JK (2018) Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J Cardiovasc Comput Tomogr 12(3):204–209
Takx RAP, Blomberg BA, Aidi HE, Habets J, Leiner T (2015) Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 8(1):e002666
Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, Diemen PA, Leipsic JA, Knuuti J, Underwood SR, van de Ven PM, van Rossum AC, Taylor CA, Knaapen P (2019) Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol 73(2):161–173
Berman DS, Kang X, Slomka PJ, Gerlach J, Yang L, Hayes SW, Friedman JD, Thomson LE, Germano G (2007) Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol 14(4):521–528
van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ (2014) Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv 7(3):301–311
Linde JJ, Hove JD, Sørgaard M, Kelbæk H, Jensen GB, Kühl JT, Hindsø L, Køber L, Nielsen WB, Kofoed KF (2015) Long-term clinical impact of coronary CT angiography in patients with recent acute-onset chest pain: the randomized controlled CATCH trial. JACC Cardiovasc Imaging 8(12):1404–1413
Newby D, Williams M, Hunter A, Pawade T, Shah A, Newby D, Forbes J, Hargreaves A, Leslie S, Lewis S, McKillop G, McLean S, Reid J, Spratt J, Uren N, Timmis A, Berry C, Boon N, Clark L, Craig P, Barlow T, Flather M, Forbes J, Lewis S, McCormack C, McLean S, Newby D, Roditi G, van Beek E, Williams M, Hunter A, Shepherd S, Bucukoglu M, Lewis S, Assi V, Parker R, Krishan A, McCormack C, Wee F, Wackett A, Walker A, Milne L, Oatey K, Neary P, Donaldson G, Fairbairn T, Fotheringham M, Hall F, Hargreaves A, Spratt J, Glen S, Perkins S, Taylor F, Cram L, Beveridge C, Cairns A, Dougherty F, Eteiba H, Rae A, Robb K, Crawford W, Clarkin P, Lennon E, Houston G, Pringle S, Ramkumar PG, Sudarshan T, Fogarty Y, Barrie D, Bissett K, Dawson A, Dundas S, Letham D, O’Neill L, Ritchie V, Weir-McCall J, Dougall H, Ahmed F, Cormack A, Findlay I, Hood S, Murphy C, Peat E, McCabe L, McCubbin M, Allen B, van Beek E, Behan M, Bertram D, Brian D, Cowan A, Cruden N, Denvir M, Dweck M, Flint L, Flapan A, Fyfe S, Grubb N, Keanie C, Lang C, MacGillivray T, MacLachlan D, MacLeod M, Mirsadraee S, Morrison A, Mills N, Northridge D, Phillips A, Queripel L, Reid J, Uren N, Weir N, Jacob A, Bett F, Divers F, Fairley K, Keegan E, White T, Fowler J, Gemmill J, McGowan J, Henry M, Francis M, Sandeman D, Dinnel L, Newby D, Bloomfield P, Denvir M, Henriksen P, MacLeod D, Morrison A, Berry C, Mangion K, Mordi I, Roditi G, Tzemos N, Connolly E, Boylan H, Brown A, Farrell L, Frood A, Glover C, Johnstone J, Lanaghan K, McGlynn D, McGregor L, McLennan E, Murdoch L, Paterson V, Teyhan F, Teenan M, Woodward R, Steedman T (2015) CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicenter trial. Lancet 385(9985):2383–2391
Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, De Bruyne B, PLATFORM Investigators (2015) Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR (CT): outcome and resource impacts study. Eur Heart J 36(47):3359–3367
Assessing and diagnosing suspected stable angina. NICE guidance. https://pathways.nice.org.uk/pathways/chest-pain#path=view%3A/pathways/chest-pain/assessing-and-diagnosing-suspected-stable-angina.xml&content=view-node%3Anodes-diagnostic-investigations. Accessed Dec 2019 17
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miyajima, K., Motoyama, S., Sarai, M. et al. On-site assessment of computed tomography-derived fractional flow reserve in comparison with myocardial perfusion imaging and invasive fractional flow reserve. Heart Vessels 35, 1331–1340 (2020). https://doi.org/10.1007/s00380-020-01606-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-020-01606-z