Abstract
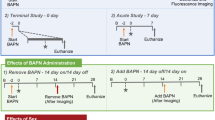
Biodegradable gelatin hydrogel sheet (BGHS) incorporating basic fibroblast growth factor (bFGF) may inhibit the progression of abdominal aortic aneurysm (AAA). We investigated whether AAA in a rat model treated with BGHS soaked with bFGF can suppress aortic expansion and recover the contractile response of aneurysmal aortic wall. Experimental AAA was induced in 10-week-old male Sprague–Dawley rats with intra-aortic elastase infusion. Aortas of these rats were assigned to 4 groups (n = 6 each) as follows: Control group, aortas infused with saline; Elastase only group, aortas infused with elastase; Hydrogel group, aortas wrapped with saline-soaked BGHS after elastase infusion; and bFGF group, aortas wrapped with bFGF (100 μg)-soaked BGHS after elastase infusion. Preoperatively and on postoperative day (POD)7 and POD14, mean aortic maximal diameter was measured ultrasonographically. Aortic expansion ratio was calculated as: (post-infusion aortic diameter on POD14/pre-infusion aortic diameter × 100). Aortas were stained with Elastica van Gieson and α-smooth muscle actin to measure the ratio of elastic fibers and α-smooth muscle actin-positive cells area to the media area. Aortas on POD14 were cut into 2-mm rings and treated with contractile agent, then tension was recorded using myography. Maximum aorta diameters were significantly greater in Elastase only group, Hydrogel group, and bFGF group than in Control group (on POD14). Maximum diameter was significantly lower in bFGF group (3.52 ± 0.4 mm) than in Elastase only group (6.21 ± 1.4 mm on POD14, P < .05). On histological analysis, ratio of the area staining positively for elastic fibers was significantly greater in bFGF group (7.43 ± 1.8%) than in Elastase only group (3.76 ± 2.9%, P < .05). The ratio for α-smooth muscle actin-positive cells was significantly lower in Elastase only group (38.3 ± 5.1%) than in Control group (49.8 ± 6.7%, P < .05). No significant differences were seen between Elastase only group and bFGF group, but ratios tended to be increased in bFGF group. Consecutive mean contractile tensions were significantly higher in bFGF group than in Elastase only group. Maximum contractile tension was significantly higher in bFGF group (1.3 ± 0.4 mN) than in Elastase only group (0.4 ± 0.2 mN, P < .05). Aortic expansion can be suppressed and contractile responses of aneurysmal aortic wall recovered using BGHS incorporating bFGF.





Similar content being viewed by others
References
Karthikesalingam A, Holt PJ, Vidal-Diez A, Bahia SS, Patterson BO, Hinchliffe RJ, Thompson MM (2016) The impact of endovascular aneurysm repair on mortality for elective abdominal aortic aneurysm repair in England and the United States. J Vasc Surg 64(2):321–327
Schanzer A, Greenberg RK, Hevelone N, Robinson WP, Eslami MH, Goldberg RJ, Louis Messina (2011) Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 123(24):2848–2855
Antoniou GA, Georgiadis GS, Antoniou SA, Neequaye S, Brennan JA, Torella F, Vallabhaneni SR (2015) Late rupture of abdominal aortic aneurysm after previous endovascular repair: a systematic review and meta-analysis. J Endovasc Ther 22(5):734–744
Takagi H, Ando T, Umemoto T (2017) Worse late-phase survival after elective endovascular than open surgical repair for intact abdominal aortic aneurysm. Int J Cardiol 236:427–431
Guessous I, Periard D, Lorenzetti D, Cornuz J, Ghali WA (2008) The efficacy of pharmacotherapy for decreasing the expansion rate of abdominal aortic aneurysms: a systematic review and meta-analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0001895
Moore G, Liao S, Curci JA, Starcher BC, Martin RL, Hendricks RT, Chen JJ, Thompson RW (1999) Suppression of experimental abdominal aortic aneurysms by systemic treatment with a hydroxamate-based matrix metalloproteinase inhibitor (RS 132908). J Vasc Surg 29(3):522–532
Hoshina K, Koyama H, Miyata T, Shigematsu H, Takato T, Dalman RL, Nagawa H (2004) Aortic wall cell proliferation via basic fibroblast growth factor gene transfer limits progression of experimental abdominal aortic aneurysm. J Vasc Surg 40(3):512–518
Miyama N, Sato A, Matsubara M, Watanabe T, Ikada Y, Satomi S (2009) Inhibitory effects of a biodegradable gelatin hydrogel sponge sheet on the progression of experimental abdominal aortic aneurysms. Ann Vasc Surg 23(2):224–230
Bartoli MA, Parodi FE, Chu J, Pagano MB, Mao D, Baxter BT, Buckley C, Ennis TL, Thompson RW (2006) Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Ann Vasc Surg 20(2):228–236
Shiraya S, Miyake T, Aoki M, Yoshikazu F, Ohgi S, Nishimura M, Ogihara T, Morishita R (2009) Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis 202(1):34–40
Zhang Q, Huang J-H, Xia R-P, Duan X-H, Jiang Y-B, Jiang Q, Sun WJ (2011) Suppression of experimental abdominal aortic aneurysm in a rat model by the phosphodiesterase 3 inhibitor cilostazol. J Surg Res 167(2):e385–e393
Lindner V, Lappi DA, Baird A, Majack RA, Reidy MA (1991) Role of basic fibroblast growth factor in vascular lesion formation. Circ Res 68(1):106–113
Sato S, Nitta Y, Saiki Y, Kawamoto S, Iguchi A, Kaku M (2008) Enhanced perigraft angiogenesis prevents prosthetic graft infection. Ann Thorac Surg 86(4):1278–1284
Iwakura A, Tabata Y, Tamura N, Doi K, Nishimura K, Nakamura T, Shimizu Y, Fujita M, Komeda M (2001) Gelatin sheet incorporating basic fibroblast growth factor enhances healing of devascularized sternum in diabetic rats. Circulation 104(90001):I325–I329
Iwakura A, Tabata Y, Koyama T, Doi K, Nishimura K, Kataoka K, Fujita M, Komeda M (2003) Gelatin sheet incorporating basic fibroblast growth factor enhances sternal healing after harvesting bilateral internal thoracic arteries. J Thorac Cardiovasc Surg 126(4):1113–1120
Azuma J, Asagami T, Dalman R, Tsao PS (2009) Creation of murine experimental abdominal aortic aneurysms with elastase. J Vis Exp 29:1280
Tabata Y, Nagano A, Ikada Y (1999) Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng 5(2):127–138
Rasband WS, ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2016. Retrieved June 22, 2017. https://imagej.nih.gov/ij/
Murakami E, Iwata H, Imaizumi M, Takemura H (2009) Prevention of arterial graft spasm by botulinum toxin: an in vitro experiment. Interact Cardiovasc Thorac Surg 9(3):395–398
Lee RT, Kamm RD (1994) Vascular mechanics for the cardiologist. J Am Coll Cardiol 23(6):1289–1295
Shimizu K, Mitchell RN, Libby P (2006) Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 26(5):987–994
Di Martino ES, Bohra A, Vande Geest JP, Gupta N, Makaroun MS, Vorp DA (2006) Biomechanical properties of ruptured versus electively repaired abdominal aortic aneurysm wall tissue. J Vasc Surg 43(3):570–576
Fujiwara H, Oda K, Saiki Y, Sakamoto N, Ohashi T, Sato M, Tabata Y, Tabayashi K (2006) The wrapping method using biodegradable felt strips has a preventive effect on the thinning of the aortic wall: experimental study in the canine aorta. J Vasc Surg 43(2):349–356
Lazarous DF, Scheinowitz M, Shou M, Hodge E, Rajanayagam MAS, Hunsberger S, Robinson WG, Stiber JA, Correa R, Epstein SE, Unger EF (1995) Effects of chronic systemic administration of basic fibroblast growth factor on collateral development in the canine heart. Circulation 91(1):145–153
Yamamoto M, Okada Y, Tabata Y (2001) Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed 12(1):77–88
Stegemann JP, Hong H, Nerem RM, Jan P (2005) Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol 98(6):2321–2327
Manderson JA, Mosse PRL, Safstrom JA, Young SB, Campbell GR (1989) Balloon catheter injury to rabbit carotid-artery. 1. Changes in smooth-muscle phenotype. Arteriosclerosis 9(3):289–298
Lysgaard Poulsen J, Stubbe J, Lindholt JS (2016) Animal models used to explore abdominal aortic aneurysms: a systematic review. Eur J Vasc Endovasc Surg 52(4):487–499
Acknowledgements
We would like to thank Kaken Pharmaceutical for providing bFGF, and also gratefully acknowledge Ms Yoshimi Hayashi and Tomoko Kamiya for their secretarial support, and Hisato Takagi, MD, PhD, for statistical advice, and we would also like to express our gratitude to the Department of General and Cardiothoracic Surgery, Graduate School of Medicine, Gifu University, for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kawai, N., Iwata, H., Shimabukuro, K. et al. Suppression of aortic expansion and contractile recovery in a rat abdominal aortic aneurysm model by biodegradable gelatin hydrogel sheet incorporating basic fibroblast growth factor. Heart Vessels 33, 793–801 (2018). https://doi.org/10.1007/s00380-017-1114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-1114-0