Abstract
Climate change has been intensifying soil drying and rewetting cycles, which can alter the soil microbiome structure and activity. Here we hypothesized that a soil drying-rewetting cycle enhances biodegradation and, hence, decreases the effectiveness of nitrification inhibitors (NIs). The effectiveness of DMPP (3,4-Dimethylpyrazole phosphate) and MP + TZ (3-Methylpyrazol and Triazol) was evaluated in 60-day incubation studies under a drying and rewetting cycle relative to constant low and high soil moisture conditions (40% and 80% water-holding capacity, WHC, respectively) in two different textured soils. The measurements included (i) daily and cumulative N2O-N emissions, (ii) soil NH4+-N and NO3−-N concentrations, and (iii) the composition of bacterial soil communities. Application of DMPP and MP + TZ reduced the overall N2O-N emissions under drying-rewetting (-45%), as well as under 40% WHC (-39%) and 80% WHC (-25%). DMPP retarded nitrification and decreased N2O-N release from the sandy and silt loam soils, while MP + TZ mitigated N2O-N production only from the silt loam soil. Unexpectedly, between days 30 and 60, N2O-N emissions from NI-treated soils increased by up to fivefold relative to the No-NI treatment in the silt loam soil at 80% WHC. Likewise, the relative abundance of the studied nitrifying bacteria indicated that the NIs had only short-term effectiveness in the silt loam soil. These results suggested that DMPP and MP + TZ might trigger high N2O-N release from fine-textured soil with constant high moisture after this short-term inhibitory effect. In conclusion, DMPP and MP + TZ effectively reduce N2O-N emissions under soil drying and rewetting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change has been causing more frequent extreme weather events, such as droughts and heavy precipitation (IPCC 2021). Consequently, soil drying and rewetting cycles have been intensified globally. These cycles can tremendously impact the emission of greenhouse gases (GHG) from agricultural lands by altering soil microbiome structure and activity (Friedl et al. 2022; Jiao et al. 2023). Soil drying may cause the breakdown of aggregates and expose protected organic C (SOC). Similarly, the release of accumulated soluble substances due to microbial cell rupture after sudden rewetting may stimulate chemical reactions and microbial activity (Liu et al. 2018). Consequently, soil rewetting might increase N2O emissions due to accumulated substrates for denitrification and enhanced microbial activity (Pezzolla et al. 2019).
The application of nitrification inhibitors (NIs) has been suggested to control N2O release from agricultural soils (Ribeiro et al. 2023). NIs can delay the oxidation of ammonium (NH4+) to nitrite (NO2−) by inhibiting the activity of ammonia-oxidizing bacteria (AOB) (Hayden et al. 2021; Ruser and Schulz 2015). In a meta-analysis considering field experiments, Yang et al. (2016) showed that the NI 3,4-Dimethylpyrazole phosphate (DMPP) efficiently delayed NH4+ conversion to nitrate (NO3−), thereby reducing its leaching and mitigating N2O release by 48%. Similarly, DMPP has been suggested to be an efficient NI under different moisture and temperature conditions (Guo et al. 2022). In addition, the NI consisting of a mixture of 3-Methylpyrazol and Triazol (MP + TZ) has been utilized in Germany and found to reduce N2O emissions (Fan et al. 2022; Guo et al. 2021; Scheer et al. 2017; Wang et al. 2020; Wu et al. 2017). Wang et al. (2020) reported that MP + TZ decreased N2O emissions by 85% and 52% from urea and urea ammonium nitrate application, respectively. Despite some studies indicating that MP + TZ can delay nitrification and mitigate N2O production (Fan et al. 2022; Pietzner et al. 2017), the impacts of this inhibitor on soil microbial communities were not investigated.
The efficiency of NIs relies on numerous factors such as temperature, moisture, pH, texture, SOC, tillage, NI, and fertilizer types and rates (Ribeiro et al. 2023). For instance, Barth et al. (2019) indicated that DMPP is less effective in clayey soils due to adsorption on soil particles and enhanced microbial activity. Nauer et al. (2018) showed that DMPP was ineffective in reducing N2O emissions from urea. Mazzetto et al. (2015) and Barneze et al. (2015) reported that N2O release from cattle urine patches in grasslands might not be reduced by NIs, probably due to warm temperatures. In addition, changes in soil moisture content are an important factor influencing NI effectiveness (Barrena et al. 2017; Di et al. 2014; Guo et al. 2022; Menéndez et al. 2012; Zhang et al. 2023). Likewise, NIs could become ineffective under soil drying and rewetting cycles due to enhanced microbial activity, which might accelerate their biodegradation. However, to the best of our knowledge, studies investigating the effect of drying and rewetting on the effectiveness of NIs are lacking.
Therefore, we hypothesized that (i) a soil drying-rewetting cycle enhances the biodegradation of NIs and weakens their nitrification inhibition effect and (ii) DMPP has a more stable effect on delaying nitrification over different soil textures and moisture conditions than MP + TZ. This study aimed to evaluate the effectiveness of DMPP and MP + TZ under a drying and rewetting cycle relative to constant low and high soil moisture conditions in coarse- and fine-textured soil. The performance of NIs was measured in terms of (i) daily and cumulative N2O-N emissions, (ii) soil NH4+-N and NO3−-N concentrations, and (iii) the composition of bacterial soil communities over the experimental period. To our knowledge, this is the first study to investigate the impacts of soil drying and rewetting on NI effectiveness.
Material and methods
Site and soil description
Sandy and silt loam soils were collected from agricultural sites in Schleswig–Holstein, northern Germany (54°00′42.9" N 8°55′43.9" E and 54°12′24" N 10°0′59" E, respectively). Subsequently, the soils were air-dried, passed through a 2 mm sieve, and homogenized. The main properties of the sandy soil were: sand, 934 g kg−1; silt, 50 g kg−1; clay, 16 g kg−1; SOC, 8.3 g kg−1; total N, 0.8 g kg−1; NH4+-N, not detected; NO3−-N, 2.93 mg kg−1; bulk density, 1.4 g cm−3; pHCaCl2, 6.2; water-holding capacity (WHC), 0.27 g g−1. The main properties of the silt loam soil were: sand, 160 g kg−1; silt, 620 g kg−1; clay, 220 g kg−1; SOC, 10 g kg−1; total N, 1.4 g kg−1; NH4+-N, 1.53; NO3−-N, 50.91 mg kg−1; bulk density, 1.3 g cm−3; pHCaCl2, 7.2; WHC, 0.35 g g−1.
Incubation experiments
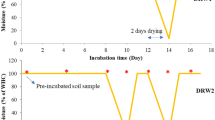
Two incubation experiments were carried out at the Institute of Plant Nutrition and Soil Science, Kiel University, Germany. These incubation experiments were conducted in a climatic chamber with 25 °C temperature and 45% relative humidity. The objective of carrying out this study under completely controlled environmental conditions was to isolate the influence of NI type and soil water content from other influencing factors. We utilized a completely randomized design with four replicates. The investigated factors were NI type (MP + TZ, DMPP, and No-NI) and soil water content (40% WHC, 80% WHC, and drying-rewetting). We utilized 40% and 80% WHC as the minimum and maximum soil moisture levels because the existing literature on NI research has adopted these as references to investigate NI performance when N2O-N emissions are expected to originate mainly from nitrification and denitrification processes, respectively (Guo et al. 2022; Torralbo et al. 2017; Zhang et al. 2023). Therefore, the same thresholds were utilized to allow the comparison of our study with the current literature. Furthermore, treatment with steady soil moisture at 60% WHC, which is the average moisture of the simulated drying-rewetting cycle, was not added to the experimental design because previous research already showed that the NIs utilized in our study are efficient at 60% WHC steady soil moisture (Guo et al. 2022; Menéndez et al. 2012; Zhang et al. 2023).
Thirty-six cylindrical pots (15 cm diameter and 33 cm length) were filled with soil up to 20 cm high. Ammonium sulfate ((NH4)2SO4) was used as N fertilizer at a 0.25 g NH4+-N kg−1 soil application rate. Both DMPP and MP + TZ were applied at 1% of the applied NH4+-N. MP + TZ was being commercialized under the name PIADIN®, which represents the combination of the active ingredients 3-Methylpyrazol (1.5%) and Triazol (3%) in urea ammonium nitrate solution. The concentration of ammonium nitrate in PIADIN® was 38% (mass), and the remaining constituents were not specified by the manufacturer (Kösler et al. 2019). Considering DMPP, the application rate of 1% of the added NH4+-N was utilized because it is within the range allowed by the European Union regulations and in agreement with the concentration used in commercial fertilizers containing DMPP (European Commission 2014). The same concentration was utilized for MP + TZ because it is within the range of application rates recommended by the manufacturer, e.g., 7 L ha−1 of the commercial product PIADIN®. The No-NI treatment received only ammonium sulfate without NI application. N fertilizer and NIs were dissolved in deionized water and surface applied on the soil surface through a 100 mL solution at the first day of incubation.
Considering the 40% and 80% WHC treatments, the soil water content was monitored daily and readjusted to the respective values by adding deionized water. For the drying-rewetting treatment, the soil water content was maintained initially at 80% of WHC at the start of the experiment (day 0). When the soil moisture content reached 40% of WHC, it was re-adjusted to 80% of WHC to simulate a drying-rewetting cycle, as demonstrated in Fig. 1.
Sampling, measurement, and calculation of N 2 O-N emissions
For N2O-N sampling, air-tight lids were placed on the pots, and the gas samples were collected with 10 mL syringes at intervals of 0, 20, 40, and 60 min. Subsequently, the samples were stored in pre-evacuated exetainers. Except for the sampling period, the pots were kept open. Gas samples were collected periodically from the headspace of the pots to measure the N2O concentration by gas chromatography (Agilent 7890A GC, Agilent). Linear interpolation was utilized to estimate N2O-N emission for the days gas sampling was not performed. Daily emissions were divided into Phase 1 (0 to 30 days of incubation) and Phase 2 (31 to 60 days of incubation) because of the different patterns of N2O-N emissions in each phase and to ease data analysis, discussion, and interpretation. Finally, the cumulative N2O-N emission was calculated from the daily emissions.
Sampling and analysis of soil NH 4 + and NO 3 −
Soil samples were collected after 15, 30, 45, and 60 days of incubation from the 0–20 cm depth for NH4+ and NO3− measurements. Considering the drying and rewetting treatment, soil samples were taken prior to soil rewetting on the 30th day of incubation. The soil samples were extracted with 1 M KCl solution (1:10 ratio) by shaking for 1 h in a shaker with a vertical rotation speed of 60 rpm. Subsequently, the suspensions were filtered, and the filtered extracts were analyzed for NH4+ and NO3− concentrations using a continuous flow analyzer (San++ Continuous Flow Analyzer, Skalar). Additionally, a sub-sample of soil was oven-dried at 105 °C for 24 h to adjust for the water content.
Soil DNA extraction, 16S rRNA gene amplicon sequencing and analysis
Samples of silt loam soil collected on the 15th and 45th day after incubation were selected for microbial community analysis, and 0.5 g of soil was used for the genomics DNA extraction using Fast DNA SPIN Kit for Soil (MP Biomedicals). Only this silt loam soil was utilized for microbial analysis to investigate the increased N2O-N emissions from NI-treated soil in Phase 2. The silt loam soil was selected because fine-textured soils present greater microbial abundance than coarse-textured ones (Guo et al. 2021).
For bacterial sequencing library preparation (in three technical replicates), primer pair targeting 16S rRNA gene V3-V4 region (341F 5’-CCTACGGGAGGCAGCAG-3’ and 806R 5’-GGACTACHVGGGTWTCTAAT-3’) was used for 16S rRNA gene amplicon sequencing. Sequencing library preparation and MiSeq paired-end (2 × 300 bp) sequencing (Illumina, v3 chemistry) on the sample pool was performed at the Center of Molecular Life Sciences (ZMB), Kiel University. ZymoBIOMICSTM Microbial Community DNA Standard was used for sequencing, data analysis, and quality control for the amplicon sequencing. 16S rRNA gene amplicon sequencing data were analyzed on the de-multiplexed fastq reads. Cutadapt (v3.5) (Martin 2011) was used for the adapter and primer removal and quality control (Q-score > 20). On average, 46,440 de-multiplexed fastq paired-end reads per sample were obtained, and 45,829 (94%) were retained after adapter trimming and quality filtering. The quality controlled paired end reads were merged using VSEARCH (v2.21.1) (Rognes et al. 2016), and 22,434 merged reads (merging percentage = 96.5%) were obtained. Chimeric sequence removal and generation of amplicon sequence variants (ASV) were performed on the merged sequences using package dada2 (v1.22.0) (Callahan et al. 2016) in RStudio (v2021.09.0 + 351) (RStudio Team 2020) running R (v4.1.3) (R Core Team 2021).
On average, 17,132 reads per sample were non-chimeric and used to analyze sequence variants, resulting in 8,295 ASVs. Taxonomic annotations of ASVs were performed using the 16S rRNA database formatted for DADA2 with Genome Taxonomy Database taxonomies (v202) (Alishum 2021). The abundance table, taxonomy table, sample metadata, and phylogenetic tree were merged into a single object and used for visualization and statistical analysis using packages phyloseq (v1.38.0) (McMurdie and Holmes 2013), vegan (v2.6.2) (Oksanen et al. 2019), ggplot2 (v3.3.6) (Wickham 2016). Out of 8,295 ASVs, ten, 8,284, and one ASV were taxonomically classified (at kingdom level) to Archaea, Bacteria, and Unknown taxa, respectively (Table 1)
Only bacterial ASVs (8,284) were considered for further analysis, and the remaining ASVs were discarded. The differential abundance testing and correlation analysis for the genera related to nitrifying bacteria was done and visualized using package DESeq2 (v1.34.0) (Love et al. 2014), stats (v4.2.2), pheatmap (1.0.12) (Kolde 2012) and ggcor (0.9.4.3) (Huang et al. 2020). MiSeq sequencing de-multiplexed paired-end fastq reads were deposited in SRA at NCBI under accession number PRJNA1018126.
Statistical analysis of N 2 O-N cumulative emissions
The data for cumulative N2O-N emissions were subjected to the normality test. Subsequently, a two-way analysis of variance (F test) was performed for the factors NI and soil moisture within each experimental phase and soil texture. The response variables that presented significant treatment effects were submitted to the Tukey test (p < 0.05) using the R software (R Core Team 2021).
Results
N 2 O-N fluxes and cumulative emissions
The overall N2O-N emission from soils was higher at 80% WHC than at drying-rewetting and at 40% WHC (Table 1, Fig. 2). Furthermore, DMPP and MP + TZ reduced the overall N2O-N release relative to No-NI by 43% and 20%, respectively (Table S1).
In the sandy soil, MP + TZ significantly reduced N2O-N emission only under drying-rewetting in Phase 1. However, this inhibitor mitigated N2O-N release under all tested moisture levels in the silt loam soil in Phase 1. DMPP significantly decreased N2O-N emissions in almost all investigated combinations of soil textures and moisture levels (Table 2). On average, applying NIs reduced N2O-N emissions by 45% under drying-rewetting, 39% under 40% WHC, and 25% under 80% WHC (Table S1). Furthermore, on average, DMPP and MP + TZ reduced N2O-N release by 58% in Phase 1 and 8% in Phase 2. The N2O-N emission from DMPP and MP + TZ treatments peaked during Phase 2 in the silt loam soil, resulting in an up to fivefold increase relative to the No-NI treatment.
Soil NH4 +-N and NO3 −-N concentrations
The nitrification delaying effect of DMPP and MP + TZ was more evident in the silt loam soil than in the sandy soil (Fig. 3). Both inhibitors delayed nitrification within the silt loam soil under all soil moisture conditions. For instance, under 40% WHC, NO3−-N reached higher concentrations than NH4+-N at 45 days after incubation in No-NI, while DMPP and MP + TZ resulted in similar NH4+-N and NO3−-N concentrations. For the drying-rewetting and 80% WHC treatments, the concentration of NO3−-N was greater than that of NH4+-N at 30 days after incubation in the No-NI treatments, while the application of DMPP and MP + TZ kept analogous levels of NO3−-N and NH4+-N.
Composition of bacterial soil communities
Application of DMPP and MP + TZ did not affect the number of reads classified as bacteria in amplicon libraries, which ranged between 15,000 and 20,000 reads for all treatments, and the Shannon index (Figures S1 and S2). However, the NIs reduced the relative abundance of the nitrifying bacteria (Fig. 4). Considering the 40% WHC, GW-Nitrospira-1 and Nitrosomonas presented higher relative abundance in No-NI than in DMPP and MP + TZ. Under drying-rewetting, the relative abundance of Nitrospira_C, Nitrospira_A, Nitrosospira, Nitrosomonas, and Nitrobacter was decreased by DMPP and MP + TZ. Additionally, Nitrosococcus, Nitrosospira, and Nitrobacter were reduced by NI application under 80% WHC.
The relative abundance of Nitrosospira and Nitrobacter was positively correlated with N2O-N emissions (Fig. 6). Soil NH4+-N concentration showed a negative correlation with Nitrospira_C and A, whereas it had a positive correlation with Nitrosococcus and Nitrobacter. Soil NO3−-N concentration was positively correlated with Nitrospira C and negatively correlated with Nitrobacter and Nitrosococcus.
In general, the relative abundance of the studied nitrifying bacteria was greater at day 15 than at day 45 in the No-NI treatment for all moisture levels. The opposite trend was shown for DMPP and MP + TZ in which the relative abundance of bacterial genera such as Nitrobacter and Nitrosococcus increased at day 45 relative to day 15 (Fig. 6).
Discussion
Soil drying and rewetting events are becoming more common due to climate change, and may decrease the effectiveness of NIs due to the enhanced microbial activity upon rewetting. The present study is the first to evaluate the performance of NIs under soil drying and rewetting. Thus, the effectiveness of DMPP and MP + TZ was evaluated under a drying and rewetting cycle relative to constant low and high soil moisture conditions in coarse- and fine-textured soils.
Short-term effect of DMPP and MP + TZ on nitrification and mitigation of N 2 O-N emissions
According to the soil mineral N concentrations, it was found that DMPP and MP + TZ delayed the nitrification process under all tested conditions (Fig. 3). In our study, autotrophic nitrification was assumed to be the main process driving the soil NO3−-N accumulation and N2O-N release because of the high NH4+-N application as ammonium sulfate. In addition, it has been suggested that NH4+-N fertilization mitigates the utilization of organic N by soil microbes (Parajuli et al. 2022). Therefore, it is improbable that heterotrophic nitrification contributed to NO3−-N and N2O-N production in this study despite its importance has been proven under different agroecosystems and soil conditions (Gao et al. 2023).
Unlike the silt loam soil, NO3−-N gradually increased while NH4+-N sharply declined in the sandy soil (Fig. 3). The NH4+-N fertilizer was applied to the soil by dissolving it in a 100 mL solution to avoid ammonia (NH3) volatilization. However, such results indicated that other N loss paths, such as NH3 volatilization, might have occurred probably because of the near-neutral pH and low cation exchangeable capacity of the sandy soil.
In the silt loam soil, DMPP and MP + TZ retarded nitrification for up to 30 days. However, after 30 days of incubation in the silt loam soil, the NO3−-N exceeded NH4+-N concentration in DMPP and MP + TZ treated soils under 80% WHC and drying-rewetting. Similarly, at 60 days after incubation, NO3−-N and NH4+-N concentrations were similar under NI-treated soils at 40% WHC. This indicated that the NIs had only short-term effects under all tested moisture levels. In contrast, Menéndez et al. (2012) and Torralbo et al. (2017) suggested that soil water content had minor effects on the persistence of DMPP in soil incubated with temperatures up to 20 °C. These contrasting results might be attributed to the higher temperature used in our study (25 °C) or distinct soil characteristics such as pH and texture.
The accumulation of NO3−-N after 30 days in the silt loam soil under 80% WHC increased N2O-N emissions by up to fivefold in DMPP and MP + TZ, respectively, relative to the No-NI treatment (Table 2). Consequently, this increased N2O-N emissions in the NI-treated soils during Phase 2 partially offset the reduced emissions during Phase 1 (Fig. 2). In contrast, DMPP and MT + TZ did not increase N2O-N release during Phase 2 in the sandy soil. Fine-textured soils commonly present higher microbial abundance and activity than coarse-textured ones (Barth et al. 2019; Guo et al. 2021), possibly decreasing NIs persistence in the silt loam soil.
It is widely known that NIs have a short-term effect as they are subjected to biodegradation. This effect may vary from 4 to 10 weeks depending on conditions such as soil temperature, texture, and pH (Zerulla et al. 2001). For instance, Menéndez et al. (2012) reported that DMPP caused a 45% and 23% reduction in N2O-N emission in soil incubated under 10 °C and 20 °C, respectively, which suggested that higher temperature reduced the overall effectiveness of DMPP. However, it has not been demonstrated yet that high soil water content could partially offset the short-term effect of NIs on reducing N2O-N release (Fig. 2). Extensive research has investigated the interaction between NIs and different moisture levels (Menéndez et al. 2012; Guo et al. 2022; Zhang et al. 2023). Torralbo et al. (2017) and Zhang et al. (2023) reported that DMPP was effective under high soil water contents. However, its efficiency was more sensitive to increased temperature under such conditions. Guo et al. (2022) reported that MP + TZ efficiently mitigated nitrification and N2O-N release under 80% WHC. In our study, the warm temperature in the incubation experiments (25 °C) probably stimulated NI biodegradation, and the hypoxic conditions under 80% WHC led to increased N2O-N release due to late NO3−-N accumulation.
Soil microbial activity and decomposition are known to have a parabolic relationship with moisture. Thus, it is expected that the biodegradation of SOC is minimal in extremely dry or wet conditions. At the same time, the highest biodegradation occurs at a soil moisture level where the water and O2 availability are optimal (Dan et al. 2016). In our study, O2 availability at 80% WHC was sufficient to promote rapid nitrification in the No-NI treatment (Fig. 3). Similarly, the greater water availability at 80% WHC might have enhanced the biodegradation of NIs relative to the 40% WHC and drying-rewetting treatments. Even if this concept is well-known and described in the literature, our study is the first to show that this process can trigger increased N2O-N emissions from NI-treated soil after 30 days. In contrast to our study, previous research found that the soil moisture did not influence or had only minor effects on NI effectiveness (Menéndez et al. 2012; Guo et al. 2022; Zhang et al. 2023). Consequently, at least for fine-textured soils with high pH under warm temperatures, our results are important to highlight that soil moisture should not be overlooked in future research or for using NIs in agriculture.
Soil texture differently affected the effectiveness of DMPP and MP + TZ
The mean reduction in N2O-N emissions caused by DMPP and MP + TZ was 43% and 20%, respectively. This decrease in N2O-N emissions by NIs is ascribed to their direct effect on nitrification and an indirect impact on denitrification due to diminished soil NO3− availability (Wu et al. 2017).
DMPP-induced decline in mean N2O-N emissions was higher for sandy soil (46%) than silt loam soil (35%). Similarly, Barth et al. (2019) suggested that DMPP is more effective for coarse than fine-textured soils, probably because of adsorption on silt and clay particles associated with the enhanced microbial activity of fine-textured soils. Conversely, the mean decline in N2O-N emissions caused by MP + TZ was lower in sandy soil (12%) than in silt loam soil (36%). In addition, MP + TZ significantly reduced N2O-N release in the sandy soil only under drying-rewetting during Phase 1. These results showed that MP + TZ had inferior performance than DMPP in the sandy soil, which might be explained by a greater MP + TZ soil mobility, which may cause leaching and spatial separation from NH4+-N in coarse-textured soils. In contrast, Guo et al. (2021) found that the inhibitory effect of MP + TZ on N2O-N release was greater in sandy than in clayey texture; however, the authors utilized a fivefold higher MP + TZ application rate than that used in this study. In addition, DMPP and NH4+-N are positively charged and, consequently, expected to present similar spatial distribution in the soil surface layers (Zerulla et al. 2001), which may explain the higher effectiveness of DMPP, especially in coarse-textured soils.
It is noteworthy that our study might have underestimated MP + TZ efficiency for two reasons. Firstly, we utilized the commercial product where the NI active ingredients were dissolved in urea ammonium nitrate solution. Therefore, the soil treated with MP + TZ received a slightly higher N rate. However, this variation is lower than 1% because the application rate of the commercial product containing MP + TZ was only 1% of the applied N. Secondly, the MP + TZ rate recommended by the manufacturer is probably based on lower N rates than the one utilized in our study. Despite this, MP + TZ delayed nitrification and reduced N2O-N emissions in the silt loam soil (Figs. 2 and 3). Finally, MP + TZ efficiency in reducing N2O-N release has been demonstrated in several studies under different environmental conditions (Fan et al. 2022; Guo et al. 2021; Pietzner et al. 2017; Scheer et al. 2017; Wang et al. 2020; Wolf et al. 2014; Wu et al. 2017).
The increased N2O-N release in NI-treated soils after 30 days of incubations was observed exclusively in the silt loam soil (Table 2, Fig. 2). This soil had 30% higher WHC than the sandy soil, which probably led to enhanced microbial activity and biodegradation of the NIs, explaining such results.
DMPP and MP + TZ temporarily reduced the relative abundance of nitrifying bacteria
The treatments presented a similar average number of reads classified as bacteria in amplicon libraries, between 15,000 and 20,000 reads, and did not impact the Shannon index (Figures S1 and S2). Similarly, Barrena et al. (2017) and Corrochano-Monsalve et al. (2021b) indicated that DMPP did not impact the total bacterial abundance and Shannon index compared to control treatments, respectively. Such results evidence that DMPP and MP + TZ specifically target the nitrifying bacteria rather than affecting the total bacterial soil community. In addition, the effect of MP + TZ on soil microbial abundance has not been reported previously.
In general, the NIs decreased the relative abundance of the nitrifying bacteria 15 days after incubation (Figs. 4 and 6). Corrochano-Monsalve et al. (2021b) found that DMPP had a specific inhibitory effect on Nitrosomonas and did not affect other nitrifying bacteria genera. Alternatively, our results indicated that the relative abundance of Nitrospira spp., Nitrosospira, Nitrosomonas, and Nitrosococcus decreased due to DMPP and MP + TZ application. Similarly, Lan et al. (2022) demonstrated that DMPP application reduced the relative abundance of Nitrosospira and Nitrosomonas from acidic and calcareous soils. Li et al. (2023) found that DMPP reduces the gene abundance of ammonia-oxidizing bacteria (AOB) from different soil types. It is noteworthy that the bacterial DNA utilized to estimate the relative abundance of nitrifying bacteria may contain DNA from dead, inactive, or low-active microbes; thus, it might provide biased evidence of the bacterial genera driving the nitrification process (Nannipieri et al. 2019). Consequently, the interpretation of the data presented in this study should take it into account.
The exact mode of action of DMPP and MP + TZ is unknown (Bozal-Leorri et al. 2022). Nevertheless, it is known that they inactivate the ammonia monooxygenase enzyme and inhibit the first step of nitrification (Corrochano-Monsalve et al. 2021a). Thus, the AOB population is commonly diminished by applying NI-treated N compared to conventional N fertilizers (Hayden et al. 2021). Accordingly, our results corroborated this premise. In addition, the positive correlation between N2O-N release and Nitrosospira and Nitrobacter suggested that nitrifying bacteria were directly or indirectly responsible for producing this GHG in our study (Fig. 5).
At day 45, the inhibitory effect of the studied NIs weakened under all investigated moisture treatments. For instance, the relative abundance of Nitrosococcus increased considerably at day 45 relative to day 15 in NI-treated soils under 40% WHC, mainly in the MP + TZ treatment (Fig. 6). These results suggest that irrespective of the soil moisture condition, the effect of DMPP and MP + TZ on mitigating nitrification was short-term. Therefore, considering the medium to long-term impacts of NI application, increased N2O-N emissions might be expected if conditions favorable to denitrification (anoxic environment, availability of organic C, and NO3−) are present regardless of anterior soil water content. In line with these results, Scheer et al. (2017) indicated that NIs can increase post-harvest N2O-N release due to late NO3− accumulation in the soil profile, which completely offset the lower emissions observed during the cropping period. Similarly, Guardia et al. (2023) reported that DMPP caused only a transitory reduction in the gene abundance of AOB in drip-irrigated soils.
Heatmap indicating the relation among the relative abundance of selected nitrifying bacterial genera and the tested treatments in an untreated (No-NI) and MP + TZ or DMPP treated silt loam soil fertilized with ammonium sulfate and subjected to different moisture conditions at 15 and 45 days after incubation
These results are crucial for managing the application of NIs in agricultural systems. For instance, the delay in nitrification caused by NIs may promote the misleading interpretation that other N management practices, such as split applications, are unnecessary. However, under high soil water content and warm temperatures, such practices should be encouraged to avoid unintended high N2O-N emissions in the medium to long-term crops. Accordingly, the N rate might also be adjusted based on the possibly higher nitrogen use efficiency promoted by NIs to prevent N surplus in soils.
Conclusions
Our findings did not support the hypothesis that soil drying and rewetting reduce the efficiency of NIs. Conversely, DMPP and MP + TZ applications effectively mitigate N2O emissions upon soil rewetting within a 30 days period. Therefore, the results evidenced that applying NIs can be a proper mitigation measure for N2O release in a scenario with more frequent soil drying and rewetting cycles due to climate change. Our second hypothesis that DMPP has a more stable effect than MP + TZ in delaying nitrification over different soil textures and moisture conditions was proved correct. MP + TZ presented a low capacity of inhibiting nitrification in the sandy soil and, therefore, it might not be recommended for coarse-textured soils, considering the conditions in which this study was carried out. In addition, this study indicated that, under favorable conditions to denitrification in the long-term, late high N2O-N emissions from fine-textured soils treated with NIs may partially offset the short-term inhibition of nitrification. This result can promote more efficient planning of NI use in agriculture once the utilization of NIs may cause the misleading interpretation that other N management practices to avoid N surplus in soils are unnecessary. Finally, this is the first study to evaluate the effectiveness of NIs under soil drying and rewetting events, which are likely to occur more commonly in the future due to climate change. Therefore, further research is still necessary to investigate the effectiveness of NIs under more intense and/or repeated drying and rewetting cycles simulating extreme weather conditions for different agroecosystems.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Alishum A (2021) DADA2 formatted 16S rRNA gene sequences for both bacteria & archaea. (Version 4.2). Zenodo. https://doi.org/10.5281/zenodo.4735821
Barneze AS, Minet EP, Cerri CC, Misselbrook T (2015) The effect of nitrification inhibitors on nitrous oxide emissions from cattle urine depositions to grassland under summer conditions in the UK. Chemosphere 119:122–129. https://doi.org/10.1016/j.chemosphere.2014.06.002
Barrena I, Menendez S, Correa-Galeote D, Vega-Mas I, Bedmar EJ, Gonzalez-Murua C, Estavillo JM (2017) Soil water content modulates the effect of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 303:1–8. https://doi.org/10.1016/j.geoderma.2017.04.022
Barth G, von Tucher S, Schmidhalter U, Otto R, Motavalli P, Ferraz-Almeida R, Meinl Schmiedt Sattolo T, Cantarella H, Vitti GC (2019) Performance of nitrification inhibitors with different nitrogen fertilizers and soil textures. J Plant Nutr Soil Sci 182:694–700. https://doi.org/10.1002/jpln.201800594
Bozal-Leorri A, Corrochano-Monsalve M, Vega-Mas I, Aparicio-Tejo PM, González-Murua C, Marino D (2022) Evidences towards deciphering the mode of action of dimethylpyrazole-based nitrification inhibitors in soil and pure cultures of Nitrosomonas europaea. Chem Biol Technol Agric 9:56. https://doi.org/10.1186/s40538-022-00321-3
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Corrochano-Monsalve M, González-Murua C, Bozal-Leorri A, Lezama L, Artetxe B (2021a) Mechanism of action of nitrification inhibitors based on dimethylpyrazole: A matter of chelation. Sci Total Environ 752:141885. https://doi.org/10.1016/j.scitotenv.2020.141885
Corrochano-Monsalve M, González-Murua C, Estavillo JM, Estonba A, Zarraonaindia I (2021b) Impact of dimethylpyrazole-based nitrification inhibitors on soil-borne bacteria. Sci Total Environ 792:148374. https://doi.org/10.1016/j.scitotenv.2021.148374
Dan WA, Nianpeng HE, Qing WA, Yuliang LÜ, Qiufeng WA, Zhiwei XU, Jianxing ZH (2016) Effects of temperature and moisture on soil organic matter decomposition along elevation gradients on the Changbai Mountains, Northeast China. Pedosphere 26:399–407. https://doi.org/10.1016/S1002-0160(15)60052-2
Di HJ, Cameron KC, Podolyan A, Robinson A (2014) Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol Biochem 73:59–68. https://doi.org/10.1016/j.soilbio.2014.02.011
European Commission (2014) Commission Regulation (EU) No 1257/2014 Amending Regulation (EC) No 2003/2003; European Parliament: Brussel, Belgium, 2014
Fan D, He W, Smith WN, Drury CF, Jiang R, Grant BB, Shi Y, Song D, Chen Y, Wang X, He P (2022) Global evaluation of inhibitor impacts on ammonia and nitrous oxide emissions from agricultural soils: A meta-analysis. Glob Chang Biol 28:5121–5141. https://doi.org/10.1111/gcb.16294
Friedl J, Deltedesco E, Keiblinger KM, Gorfer M, De Rosa D, Scheer C, Grace PR, Rowlings DW (2022) Amplitude and frequency of wetting and drying cycles drive N2 and N2O emissions from a subtropical pasture. Biol Fertil Soils 58:593–605. https://doi.org/10.1007/s00374-022-01646-9
Gao W, Fan C, Zhang W, Li N, Liu H, Chen M (2023) Heterotrophic nitrification of organic nitrogen in soils: process, regulation, and ecological significance. Biol Fertil Soils 59:261–274. https://doi.org/10.1007/s00374-023-01707-7
Guardia G, García-Gutiérrez S, Vallejo A, Ibáñez MA, Sanchez-Martin L, Montoya M (2023) Nitrous oxide emissions and N-cycling gene abundances in a drip-fertigated (surface versus subsurface) maize crop with different N sources. Biol Fertil Soils 29:1–7. https://doi.org/10.1007/s00374-023-01791-9
Guo Y, Naeem A, Mühling KH (2021) Comparative effectiveness of four nitrification inhibitors for mitigating carbon dioxide and nitrous oxide emissions from three different textured soils. Nitrogen 2:155–166. https://doi.org/10.3390/nitrogen2020011
Guo Y, Naeem A, Becker-Fazekas S, Pitann B, Mühling KH (2022) Efficacy of four nitrification inhibitors for the mitigation of nitrous oxide emissions under different soil temperature and moisture. J Plant Nutr Soil Sci 185:60–68. https://doi.org/10.1002/jpln.202000367
Hayden HL, Phillips LA, Marshall AJ, Condon JR, Doran GS, Wells GS, Mele PM (2021) Nitrapyrin reduced ammonia oxidation with different impacts on the abundance of bacterial and archaeal ammonia oxidisers in four agricultural soils. Appl Soil Ecol 157:103759. https://doi.org/10.1016/j.apsoil.2020.103759
Huang H, Zhou L, Chen J, We T (2020). ggcor: Extended tools for correlation analysis and visualization. https://github.com/houyunhuang/ggcor. Accessed 04 June 2023
IPCC (2021) Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st edn. Cambridge University Press
Jiao P, Yang L, Nie X, Li Z, Liu L, Zheng P (2023) Dependence of cumulative CO2 emission and microbial diversity on the wetting intensity in drying-rewetting cycles in agriculture soil on the Loess Plateau. Soil Ecol Lett 5:220147. https://doi.org/10.1007/s42832-022-0147-1
Kolde R (2012) Pheatmap: pretty heatmaps. Comprehensive R Archive Network (CRAN). https://cran.r-project.org/package=pheatmap. Accessed 04 June 2023
Kösler JE, Calvo OC, Franzaring J, Fangmeier A (2019) Evaluating the ecotoxicity of nitrification inhibitors using terrestrial and aquatic test organisms. Environ Sci Eur 31:91. https://doi.org/10.1186/s12302-019-0272-3
Lan T, Li M, He X, Deng O, Zhou W, Luo L, Chen G, Yuan S, Ling J, Zeng M, Gao X (2022) Effects of synthetic nitrification inhibitor (3, 4-dimethylpyrazole phosphate; DMPP) and biological nitrification inhibitor (methyl 3-(4-hydroxyphenyl) propionate; MHPP) on the gross N nitrification rate and ammonia oxidizers in two contrasting soils. Biol Fertil Soils 58:333–344. https://doi.org/10.1007/s00374-022-01628-x
Li Z, Xu P, Han Z, Wu J, Bo X, Wang J, Zou J (2023) Effect of biochar and DMPP application alone or in combination on nitrous oxide emissions differed by soil types. Biol Fertil Soils 59:123–138. https://doi.org/10.1007/s00374-022-01688-z
Liu S, Schloter M, Brüggemann N (2018) Accumulation of NO2− during periods of drying stimulates soil N2O emissions during subsequent rewetting: Nitrite stimulates N2O emissions during rewetting. Eur J Soil Sci 69:936–946. https://doi.org/10.1111/ejss.12683
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet j 17:10. https://doi.org/10.14806/ej.17.1.200
Mazzetto AM, Barneze AS, Feigl BJ, Van Groenigen JW, Oenema O, de Klein CA, Cerri CC (2015) Use of the nitrification inhibitor dicyandiamide (DCD) does not mitigate N2O emission from bovine urine patches under Oxisol in Northwest Brazil. Nutr Cycl Agroecosystems 101:83–92. https://doi.org/10.1007/s10705-014-9663-4
McMurdie PJ, Holmes S (2013) phyloseq: An r package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217. https://doi.org/10.1371/journal.pone.0061217
Menéndez S, Barrena I, Setien I, González-Murua C, Estavillo JM (2012) Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biol Biochem 53:82–89. https://doi.org/10.1016/j.soilbio.2012.04.026
Nannipieri P, Penton CR, Purahong W, Schloter M, Van Elsas JD (2019) Recommendations for soil microbiome analyses. Biol Fertil Soils 55:765–766. https://doi.org/10.1007/s00374-019-01409-z
Nauer PA, Fest BJ, Visser L, Arndt SK (2018) On-farm trial on the effectiveness of the nitrification inhibitor DMPP indicates no benefits under commercial Australian farming practices. Agric Ecosyst Environ 253:82–89. https://doi.org/10.1016/j.agee.2017.10.022
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'hara RB, Simpson GL, Solymos P, Stevens MH (2019) vegan: Community Ecology Package. Comprehensive R Archive Network (CRAN). https://cran.r-project.org/package=vegan. Accessed 19 June 2023
Parajuli B, Ye R, Szogi A (2022) Mineral N suppressed priming effect while increasing microbial C use efficiency and N2O production in sandy soils under long-term conservation management. Biol Fertil Soils 58:903–915. https://doi.org/10.1007/s00374-022-01665-6
Pezzolla D, Cardenas LM, Mian IA, Carswell A, Donovan N, Dhanoa MS, Blackwell MS (2019) Responses of carbon, nitrogen and phosphorus to two consecutive drying–rewetting cycles in soils. J Plant Nutr Soil Sci 182:217–228. https://doi.org/10.1002/jpln.201800082
Pietzner B, Rücknagel J, Koblenz B, Bednorz D, Tauchnitz N, Bischoff J, Köbke S, Meurer KH, Meißner R, Christen O (2017) Impact of slurry strip-till and surface slurry incorporation on NH3 and N2O emissions on different plot trials in Central Germany. Soil Tillage Res 169:54–64. https://doi.org/10.1016/j.still.2017.01.011
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria. http://www.r-project.org. Accessed 11 May 2023
Ribeiro PL, Carlos FS, Barth G, Mühling KH (2023) Do tropical climatic conditions reduce the effectiveness of nitrification inhibitors? A meta-analysis of studies carried out in Brazil. Nutr Cycl Agroecosystems 125:345–358. https://doi.org/10.1007/s10705-023-10266-0
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. https://doi.org/10.7717/peerj.2584
RStudio Team (2020) RStudio: Integrated Development Environment for R. Boston, MA: RStudio, PBC, http://www.rstudio.com. Accessed 11 May 2023
Ruser R, Schulz R (2015) The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—a review. J Plant Nutr Soil Sci 178:171–188. https://doi.org/10.1002/jpln.201400251
Scheer C, Rowlings D, Firrell M, Deuter P, Morris S, Riches D, Porter I, Grace P (2017) Nitrification inhibitors can increase post-harvest nitrous oxide emissions in an intensive vegetable production system. Sci Rep 7:43677. https://doi.org/10.1038/srep43677
Torralbo F, Menéndez S, Barrena I, Estavillo JM, Marino D, González-Murua C (2017) Dimethylpyrazol-based nitrification inhibitors effect on nitrifying and denitrifying bacteria to mitigate N2O emission. Sci Rep 7:13810. https://doi.org/10.1038/s41598-017-14225-y
Wang H, Köbke S, Dittert K (2020) Use of urease and nitrification inhibitors to reduce gaseous nitrogen emissions from fertilizers containing ammonium nitrate and urea. Glob Ecol Conserv 22:e00933. https://doi.org/10.1016/j.gecco.2020.e00933
Wickham H (2016) Data Analysis. In: ggplot2. Use R!. Springer, Cham. https://doi.org/10.1007/978-3-319-24277-4_9
Wolf U, Fuß R, Höppner F, Flessa H (2014) Contribution of N2O and NH3 to total greenhouse gas emission from fertilization: results from a sandy soil fertilized with nitrate and biogas digestate with and without nitrification inhibitor. Nutr Cycl Agroecosystems 100:121–134. https://doi.org/10.1007/s10705-014-9631-z
Wu D, Senbayram M, Well R, Brüggemann N, Pfeiffer B, Loick N, Stempfhuber B, Dittert K, Bol R (2017) Nitrification inhibitors mitigate N2O emissions more effectively under straw-induced conditions favoring denitrification. Soil Biol Biochem 104:197–207. https://doi.org/10.1016/j.soilbio.2016.10.022
Yang M, Fang Y, Sun D, Shi Y (2016) Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: a meta-analysis. Sci Rep 6:22075. https://doi.org/10.1038/srep22075
Zerulla W, Barth T, Dressel J, Erhardt K, Horchler von Locquenghien K, Pasda G, Rädle M, Wissemeier A (2001) 3,4-Dimethylpyrazole phosphate (DMPP) - a new nitrification inhibitor for agriculture and horticulture. Biol Fertil Soils 34:79–84. https://doi.org/10.1007/s003740100380
Zhang X, Xintong Xu, Chenyuan Wa, Zhang Q, Yubing Do, Xiong Z (2023) DMPP mitigated N2O and NO production by inhibiting ammonia-oxidizing bacteria in an intensified vegetable field under different temperature and moisture regimes. Pedosphere. https://doi.org/10.1016/j.pedsph.2023.03.018
Acknowledgements
PL Ribeiro acknowledges the support from the German Academic Exchange Service (DAAD) Doctoral Research Grant (number 57507871). A Naeem is thankful to the Alexander von Humboldt Foundation for the George Forster Post-Doctorate Fellowship grant.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Pablo Lacerda Ribeiro and Karl Hermann Mühling contributed to the study conception and design. Material preparation, data collection and analysis were performed by Pablo Lacerda Ribeiro, Abhijeet Singh, Amit Sagervanshi and Asif Naeem. The first draft of the manuscript was written by Pablo Lacerda Ribeiro and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Karl Hermann Mühling performed the project administration and funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ribeiro, P.L., Singh, A., Sagervanshi, A. et al. High soil moisture rather than drying-rewetting cycles reduces the effectiveness of nitrification inhibitors in mitigating N2O emissions. Biol Fertil Soils 60, 627–638 (2024). https://doi.org/10.1007/s00374-024-01811-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-024-01811-2