Abstract
The combination of conservation tillage (non-inversion and no-till) with organic farming is rare due to weed problems. However, both practices have the potential to improve soil quality and increase soil organic C (SOC). This study investigated the changes in SOC, microbial biomass, and microbial composition during the transition from conventional to organic farming (from 2014 to 2020) in a long-term tillage trial established in 1999. Non-inversion minimum tillage to a depth of 10 cm (MT) resulted in SOC stratification, whilst conventional soil tillage with 25-cm-deep mouldboard ploughing (CT) maintained an even SOC distribution in the plough layer. After 12 years of contrasting tillage in 2011, the uppermost soil layer under MT had a 10% higher SOC content (1.6% w/w) than CT (1.45% w/w). This difference became even more pronounced after introducing organic farming in 2014. By the fall of 2020, the SOC content under MT increased to 1.94%, whilst it decreased slightly to 1.36% under CT, resulting in a 43% difference between the two systems. Conversion to organic farming increased microbial biomass under both tillage systems, whilst SOC remained unchanged in CT. Abundances of total bacterial and Crenarchaeal 16S rRNA and fungal ITS genes indicated shifts in the microbial community in response to tillage and depth. Fungal communities under MT were more responsive to organic farming than bacterial communities. The improved soil quality observed under MT supports its adoption in both organic and conventional systems, but potentially large yield losses due to increased weed cover discourage farmers from combining MT and organic farming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conventional agricultural practices, including intensive tillage, fertilisation, monocropping, and the use of pesticides, have raised significant environmental concerns, including nutrient losses, greenhouse gas emissions, and biodiversity decline. To address these challenges, there is a need for agricultural practices that enhance environmental sustainability and resource use efficiency, thereby reducing the negative impact on the environment. Conventional tillage, which involves ploughing and subsequent soil preparation for sowing or planting, plays a significant role in seed emergence, early crop development, and weed control (Hobbs et al. 2008). However, long term, it can lead to soil erosion and a decline in soil organic matter due to accelerated soil organic matter (SOC) mineralisation (Montgomery 2007; Six et al. 1999). Moreover, tillage indirectly affects SOC transformations by disrupting soil aggregates and altering the activity and composition of soil microbiota, which in turn affects vital ecosystem functions such as mineralisation and nutrient retention (Bowles et al. 2014).
Non-inversion tillage methods, such as reduced or minimum tillage (MT) and no-tillage (NT), have emerged as alternatives to traditional ploughing practices (Hobbs et al. 2008; Mäder and Berner 2012). MT and NT have been shown to positively affect soil properties by increasing SOC in the topsoil layer (Haddaway et al. 2017). Additionally, higher microbial biomass and activity in the upper soil layer were reported (Heinze et al. 2010; Kaurin et al. 2015; Sauvadet et al. 2018). However, non-inversion tillage and NT are predominantly utilised in conventional crop production, rarely in organic farming systems (Krauss et al. 2020).
Various soil management practices, such as residue return, cover cropping, crop rotation, and organic fertilisation, have been discussed within the context of organic farming system to improve soil quality (Watson et al. 2002; Fließbach et al. 2007; Tuomisto et al. 2012) and increase of SOC stocks (Gattinger et al. 2012). Assuming that reduced tillage intensity already offers many environmental benefits, integrating it into organic farming may enhance these benefits, albeit with some adjustments to the system components (Mäder and Berner 2012). However, the adoption of reduced tillage methods in organic systems often leads to increased weed problems since synthetic herbicides are not allowed (Casagrande et al. 2016). Conventional tillage, trough soil inversion, decreases the weed seed concentration on the topsoil and creates less favourable conditions for weed succession (Sans et al. 2011; Krauss et al. 2020). Consequently, limited knowledge exists about the interaction between reduced tillage intensity and organic farming, and many questions remain unanswered regarding the successful implementation of reduced tillage in organic farming (Peigné et al. 2007; Krauss et al. 2020).
Only a few long-term studies in Europe have investigated the effects of non-inversion, reduced tillage in organic farming (Sun et al. 2016; Krauss et al. 2017, 2020; Loaiza Puerta et al. 2019). Therefore, further research is needed to cover different pedo-climatic environments that significantly moderate the outcomes. Moreover, the performance of non-inversion minimum tillage relative to ploughing during the transition from conventional to organic crop production system has been rarely studied. Only one relevant reference was found, suggesting that using reduced tillage during the organic transition can increase soil quality without compromising yield and profitability (Lewis et al. 2011). Studying the transition period is critical for understanding the impacts on yields and soil quality, considering more extensive weed cover and its effects on yields as well as soil C inputs and dynamics.
The objective of this study was to compare two tillage methods: non-inversion minimum tillage (MT) and. conventional ploughing (CT), during the 5-year transition from conventional to organic farming. It is important to underline that the two tillage practices have been conducted side-by-side, employing the same agro-technical measures, for a continuous 20-year duration within long-term field experiment. We started a comparison of MT and CT under conventional management in 1999, and subsequently, both tillage systems were converted to organic farming in 2014, rendering our experiment distinctly unique. Soil quality parameters, yields, and weed dynamics have been monitored since the start of the experiment in 1999, with more regular measurements during the crucial 5-year transition period to the organic farming system (2015–2020). We hypothesised that minimum tillage in organic farming would further enhance soil C sequestration, microbial biomass, and abundance of the total microbial community (bacteria, archaea, fungi) compared to conventional ploughing.
Materials and methods
Field site, experiment design, and historical management
Long-term tillage experiment (LTE) Moškanjci was established in 1999 and transitioned from conventional to organic agriculture in 2014. The field experiment is located in the Subalpine south geo-region (Metzger 2018), Slovenia (46°3′, 15°4′; 225 m a.s.l.) and compares two tillage systems (conventional plough tillage (CT) vs. non-inversion minimum tillage (MT)), based on a plot trial with ten replicated plots for each tillage treatment. The climate is continental with an average annual temperature of 10.9 °C and precipitation of 926 mm for the period 1999–2021 (ARSO 2022). The soils are classified as Eutric Cambisols, with a loamy texture. At the beginning of the experiment in 1999, the SOC content was 1.40% and pH 6.7.
Prior to conversion to organic agriculture, conventional practices were employed, including fertilisation and pesticide usage. In general, summer crops such as maize, sugar beet, and sunflower were fertilised either with pig or cattle slurry (20–30 t/ha, approximately 120 to 170 kg N/ha) in the spring, and supplemented with starter NPK at sowing/planting. Winter cereals and w. oil rape were fertilised exclusively with mineral fertilisers. Crops were side dressed once in a crop vegetative development phase with mineral N (CAN or urea). After harvests, straw remained in the field. Herbicides, fungicides, and insecticides were used in accordance with best management practice at the time, following the principles of integrated pest management. After conversion to organic management, fertilisation shifted exclusively to organic fertilisers, primarily cattle slurry, and occasionally cattle manure. The organic fertilisation is now applied once per year in the spring at a rate of approximately 30 t/ha (with approximately 7% DM in slurry), providing 100–140 kg N/ha, 20–25 kg P/ha, and 90–120 kg K/ha. Due to previous conventional fertilisation, the soil still retains high levels of plant available phosphorus and potassium. Prior to conversion to organic agriculture, the management of broad-leaved weeds and grasses in crops like maize, sunflower, and sugar beet involved the application of herbicides, including dimethenamid, pendimethalin, and bentazone as active ingredients, whilst in winter cereals weeds were controlled using post-emergence herbicide iodosulfuron-methyl-sodium (Table S8). Additionally, systemic fungicides thiophanate methyl and epoxiconazole were used to combat foliar diseases in sugar beet, whilst broad spectrum contact and systemic fungicides were used in winter cereals (e.g. iodosulfuron-methyl-sodium). Insecticides were never used, even during conventional farming on experimental site. No pesticides have been used since the conversion to organic system. The weed control is achieved through tine combing and harrowing by inter-row cultivators later in the growing season, as well as introduction of cover crops. After conversion to organic farming, a strong emphasis has been placed on year-round soil cover with crops.
Soil sampling
Soil samples were collected regularly in late autumn to minimise seasonal influences. Only the years in which all relevant analyses were conducted for the scope of this study were included (2011, 2015, 2016, 2017, 2019, 2020). Samples were taken from four replicated plots per treatment; each sample in one plot was composed of 5 cores. Soil samples were collected from three soil layers: 0–10 cm, 10–20 cm, and 30–60 cm. The layer between 20 and 30 cm was not sampled, because it is a transitional layer between the ploughing depth and the subsoil (typically ploughed to a depth of approximately 25 cm). The collected soil samples were thoroughly mixed to ensure homogeneity and passed through a 2-mm mesh sieve to remove roots and stones. Once samples were homogenised, they were divided into three parts for different analyses. One part of the homogenised soil sample was air-dried in a fan chamber at 40 °C for 24 h and used for chemical analysis. The second part was immediately placed on dry ice and stored at −20 °C for subsequent molecular analyses. The third part was used for soil dry matter determination.
Soil analysis
Soil properties
For soil chemical analyses, samples were air-dried and sieved to 2 mm (ISO11464, 2006). Soil organic C (SOC) and total N (TN) were determined by dry combustion (ISO 10694, 1996; ISO 13878, 1987) using an elemental analyser (Elementar vario MAX instrument, Germany). Dissolved organic C (DOC), dissolved organic N (DON), nitrate N (NO3−-N), and ammonium N (NH4+-N) were extracted with 0.01 M CaCl2 solution (ISO 14055, 1999) and determined using vario TOC cube (Elementar, Germany) for DOC and DON, and Gallery Automated Photometric Analyzer (Thermo Scientific) for NO3−-N and NH4+-N detection. Plant-available phosphorus (P) and potassium (K) were determined after ammonium lactate extraction according to Egner-Riehm-Domingo (Egner et al. 1960). Carbonates were determined after soil reaction with HCl (ISO 10693, 1995) and soil texture by the pipette method (ISO 11277, 2009). Soil pH was measured in a 1/2.5 (w/v) ratio of soil and 0.01 M CaCl2 suspension (ISO 10390, 2005). Soil water content was determined by oven drying at 105 °C to constant weight (ISO 11465, 1993).
Soil organic C stocks
For the estimation of SOC stocks, soil bulk density (ρb) was determined in two depths (0–10, 10–20 cm) using soil cylinders (ISO 11272, 1993). SOC stocks were calculated from SOC concentrations in the period 2011–2020 and measured bulk densities (2019) per soil layer as
with SOC stock in t ha−1, SOC contents in kg t−1, ρb in g dry matter soil cm−3, and h is the layer thickness (m).
SOC stocks per layer were then summed up to total SOC stocks in 0–20 cm.
Yields, crop biomass, and weed abundance
Crop total above-ground biomass and yield were determined by harvesting a selected area at a technological ripeness of crops in 4 to 6 replicates per treatment. The size of the plots was adapted to the crop species. For cereals, canola, and bean, a 1.5 × 1.5-m area was used for biomass sampling, whereas we sampled two rows 2 m long for maize. In 2019 and 2020, the weed biomass was sampled in parallel on the same sampling plots as the crop after the crop plants were first removed. Crop and weed biomass were then oven dried to air dryness (45 °C, 5 days). The grain yield was extracted from crop biomass using threshing or manual grain separation from the corn cob. Air-dried biomass and grain/seed yield were weighed and expressed in kg ha−1. The subsample of each biomass component (straw, grain, weeds) was cut and grinded to a final fineness of 0.25–10 mm using Retsch cutting mill and subjected to chemical analyses (N, P, K, C). Weed cover was additionally estimated by ocular estimation at different stages of plant growth. Weed community composition was also determined using a vegetation sampling approach according to Braun-Blanquet (1964) (data not shown here). Weed cover data are from 2017 on, 2 years after the introduction of the organic farming system.
Soil microbial biomass
Microbial biomass C (Cmic) was determined by the chloroform fumigation extraction method, adapted from Joergensen and Brookes (2005). In brief, 8 g moist soil samples were fumigated with 20 mL of ethanol-free-chloroform (CHCl3) for 24 h, and then 8 g fumigated, and a separate 8 g non-fumigated sample was extracted with 40 mL 0.01 M CaCl2 solution. Organic C in the extracts was measured using Vario TOC cube (Elementar, Germany). Cmic was calculated using a kEC value of 0.45 (Joergensen 1996).
DNA extraction and quantitative real-time PCR
DNA was extracted from 0.25 g of fresh soil using the Power Soil DNA Isolation Kit (MoBIO Laboratories, Carlsbad, CA, USA) after a 10-min homogenisation on Mini-Beadbeater-8 (BipSpec, USA). Extracted DNA was quantified by NanoDrop 2000 UV-Vis spectrophotometer (NanoDrop 2000, Thermo Scientific, Waltham, MA, USA) and stored at −20 °C until use.
Quantitative real-time PCR (qPCR) was used to quantify bacterial and Crenarchaeal 16S rRNA genes (Muyzer et al. 1993; Ochsenreiter et al. 2003) and fungal ITS genes (White et al. 1990). Reactions were carried out in a 15 μL reaction volume containing 7.5 μL Absolute Blue QPCR SYBR Green Rox mix (Thermo Scientific), 1.5 μL of each primer (10 μM), 0.5 μL of T4gp32 (500 ng/μL) (MP Biomedicals, ZDA), and 2 μL of DNA template (1 ng/μL) using 7500 Fast Real-Time PCR System v2.0.4 (Applied Biosystems, Carlsbad, CA, USA). All primers and PCR conditions are described in the supplementary material (Table S1). Standard curves were obtained using serial dilutions of plasmid standards of respective functional genes ranging from 107 to 102 copies per reaction. Plasmid standards obtained from transformed Escherichia coli cells (strain JM109) with inserted plasmid PGEM-T (Promega, Madison, WI, USA) were used. The presence of PCR inhibitors in DNA samples was performed by diluting and spiking soil DNA with a known amount of an exogenous external control (the pGEM-T vector, Promega). In all cases, inhibition was not detected. Melting curves were analysed for all runs to ensure PCR specificity.
Statistical analysis
The effect of tillage treatments, soil depth, and time of sampling on measured soil properties (SOC, Cmic, abundance of microbial groups) were analysed using linear mixed-effect models with plots within each tillage treatment used as random effects. Tillage, soil depth, time, and their interactions were considered as fixed effects. For deeper insight, subsets of data were analysed again using linear mixed models either to compare tillage, soil depth, and their interaction effects for each sampling date or to compare temporal dynamics of a selected variable between tillage treatments for each soil depth layer.
For weed cover data, a mixed model was used with treatment and time as fixed-effect factors and replicated plot within each tillage as a random factor. For each weed cover assessment date, tillage treatments were additionally compared using planned contrasts. For yield data, the yield of each crop was compared between tillage treatments using t-test.
For all models, the assumptions of normality and homoscedasticity were assessed graphically (Q-Q plot and boxplots of intercepts and slopes, respectively). In all tests, a 0.05 significance level was used. Data analysis and graphing were performed using R environment (packages nlme and emmeans for models and ggplot2 for graphing) (R Core Team 2021).
Results
Crop yields and weed cover during the transition to organic farming in dependence of tillage
On average over 20 years, the relative yields observed under MT were 94.9% of those under CT. However, individual crop yield differences between the tillage treatments were generally not significant (Table 1), even after the conversion to organic farming. The only instances of significantly lower yields in MT compared to CT occurred in the earliest years of the experiment. Notably, maize yields in 2021 indicated an advantage for CT (marginally significant). Comparing yields before and after conversion to organic farming is difficult due to the relatively small dataset and substantial yield variability of the same crop species across different years, influenced by weather effects combined with pest occurrences. Nevertheless, it is worth mentioning that the yields of winter rye in 2020 and maize in 2021 were the lowest on record for these respective crop species, excluding the extremely dry year of 2003.
It should be noted that after the conversion to organic farming we encountered difficulties in measuring certain yields, and in some cases, we had to assess the whole aboveground biomass prior to crop maturity, as indicated in Table 1. This was due to rapid and extensive weed infestations, primarily in summer crops, which dictated the early crop termination before its maturity, to prevent a substantial increase in the weed seed bank. Unfortunately, neither weed cover nor weed biomass were measured before the conversion to organic farming, which precludes to numerically compare weed problematics before and after the conversion. But we can state that the herbicide usage during the conventional period successfully controlled weeds, preventing them from being a significant factor affecting crop growth and yield. Consequently, no crop termination due to weeds was necessary in that period.
During the period of implementing organic farming, significantly larger weed cover was observed in the MT plots compared to CT plots (Fig. 1). The differences in weed cover between CT and MT were most pronounced during the early growth stages of crops and cover crops, with a substantial increase in weed cover observed in MT, but not in CT. This indicates that ploughing had a more significant effect on weed mortality and the concentration of weed seeds in the topsoil. An exception was observed in 2019 with beans, which were planted after the roller-crimping of pre-crop rye at the anthesis stage (beginning of June). The roller-crimped rye was retained as a soil cover in MT or mulched and ploughed into the soil in CT. In this particular case, weed cover in late June, early July was significantly larger in CT than in MT, possibly due physical and the allelopathic effects of surface mat of rye straw at MT, which inhibited weed germination. However, the final weed biomass measurements in 2019 and 2020 did not significantly differ between MT and CT. It was 2.6 t/ha for both MT and CT in 2019, and 2.9 t/ha and 3.2 t/ha in 2020, respectively.
Weed cover estimates for two tillage treatments in different crops after the adoption of organic principles in LTE Moškanjci. Boxplots are shown for each sampling and each tillage treatment (n = 6). For clarity, boxplots of the same sampling time are shifted slightly. Asterisks (*) denote significant differences between treatments for each sampling time at 0.05 level
Effect of soil management on soil properties
Plant-available phosphorus (P) was affected by the tillage system (p < 0.0001), soil depth (p = 0.0083), and sampling time (p < 0.0001), whilst the content of plant-available potassium (K) was affected by tillage system only in the interaction with soil depth and sampling time (p < 0.0001) (Table S3). The contents of P and K were significantly higher in the upper soil layer 0–10 cm in MT than in CT (Table 2). Average P content did not differ significantly in CT between the depths of 0–10 and 10–20 cm during the entire period of measurements, whilst in MT we observed a significant difference between the upper depths. Similarly, K content in CT between the depths of 0–10 and 10–20 cm did not differ significantly throughout the measurement period, except for December 2019, when the average K content was significantly higher in the 10–20-cm soil layer than in the 0–10 cm. In MT, K content was significantly higher in the upper soil layer 0–10 cm than 10–20 cm during the entire period (Table 2).
The NO3−-N content was significantly affected by the tillage system only in interaction with soil depth and sampling time (p < 0.0001), whilst NH4+-N content was significantly influenced only by depth (p < 0.0001) and sampling time (p < 0.0001) (Table S4). Both the NO3−-N content and the NH4+-N content varied over the years (Table 3). In general, NO3−-N content was the highest in the topsoil layer 0–10 cm under MT relative to CT. In some years, the average NO3−-N contents were even higher at a 30–60-cm depth than at a depth of 0–10 cm, which applies above all to the CT treatment.
No statistically significant differences in soil pH between the tillage systems were observed (Table 2; Table S3).
Effect of soil management on soil organic C
Soil organic C (SOC) content was affected by tillage system (p < 0.0001), soil depth (p = 0.0562), and sampling time (p = 0.0029) (Table S3). The SOC content in the upper 0–10 cm has been afterwards increasing under MT (e.g. 1.60% in 2011; 1.94% in 2020), whilst it remained at the same level under CT (1.45% in 2011, 1.36% in 2020). Significant differences were also not observed in the lower 10–20-cm soil layer at any of the time points (p < 0.05) (e.g. SOC at a depth of 10–20 cm was 1.40% and 1.45% in 2020 under CT and MT, respectively). The C/N ratio was affected by soil depth (p = 0.0002) and sampling time (p < 0.0001), whereas no difference was found between the tillage systems (Table S3).
It is important to emphasise that the accumulation of SOC was slow and reached significant differences between MT and CT in the upper 0–10-cm layer only after 12 years of contrasting tillage. The transition to organic farming resulted in a more significant increase in SOC in the upper 0–10 cm under MT, e.g. from 1.63% in 2015 to 1.94% in 2020. In contrast, no differences were found in SOC in the deeper soil layers (10–20 cm and 30–60 cm). Interestingly, the transition to organic farming had no significant effect on SOC concentrations under CT (Fig. 2). In terms of C stocks, MT increased C stocks in the upper 20-cm soil layer averaging from 40.6 to 46.6 t/ha in 5 years after the transition, whilst under CT SOC stocks decreased from 43.4 t/ha (year 2015) to 41.4 t/ha (year 2020) (Table 4).
Effect of soil management on soil microbial biomass
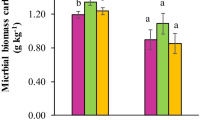
Soil microbial biomass C (Cmic) was affected by the tillage system (p = 0.0044), soil depth (p < 0.001), and sampling time (p < 0.001) (Table S5). Transition to organic farming resulted in a significant increase in Cmic in the upper soil layer (0–10 cm) under both tillage treatments (p < 0.001) (Fig. 3). The increase was higher under MT, where Cmic in the upper 0–10 cm increased from 173 to 494 mg/kg of soil after 5 years of organic farming. In contrast, CT showed lower Cmic levels, which increased from 96 to 254 mg/kg in the upper 10 cm during the same 5-year transition period. Under both tillage treatments, the significant increase in Cmic levels was observed in the 10–20-cm layer as well (from 93.6 to 292.9 mg/kg and 85.9 to 311.2 mg/kg, respectively, for MT and CT) 5 years after the transition, whilst in the deepest soil layer of 30–60 cm, the slight increase was observed only for MT (from 22 to 90 mg/kg), but not under CT (Fig. 3); however, both were statistically insignificant (p = 0.1214) (Table S6).
Changes in Cmic, influenced by tillage, were also reflected in the ratio of microbial biomass-C to soil organic C (Cmic:SOC), which was significantly affected by the tillage system (p < 0.0001), soil depth (p < 0.0001), and sampling time (p < 0.0001) (Tables S5, S7). The interactions between soil depth and tillage (p < 0.0001) and soil depth and sampling time (p < 0.01) were also significant. The ratio was significantly higher in the upper 10 cm of the MT compared to CT throughout the entire period after the transition to the organic farming (2015–2020). In the 10–20-cm soil layer, no significant differences were found in the ratio between MT (2.0%) and CT (2.2%). Five years after the transition (year 2020), the significantly higher Cmic:SOC ratio was observed at both depths (0–10 cm and 10–20 cm) for both tillage systems (CT and MT), compared to the first year of the transition (year 2015).
Effect of soil management on the abundance of the total bacterial, archaeal, and fungal communities
Abundances of bacterial and archaeal 16S rRNA genes and fungal ITS genes were affected by the tillage system (p < 0.05), soil depth (p < 0.05), and sampling time (p < 0.01) (Table S5) and have been significantly higher under MT than under CT in the upper 0–10 cm at all time points from the first molecular analyses in 2011 to 2020 (Fig. 4). After the transition to organic farming, there was a visible trend towards an increase in the abundance of all genes over the 5 years, but only in the upper 0–10 cm under MT, where the abundance of bacteria, archaea, and fungi increased from 2.17 × 109 to 5.26 × 109, 4.39 × 107 to 1.45 × 108, and 2.16 × 108 to 6.08 × 108, respectively. In contrast, there was no change under CT in this transition period. The bacteria-to-fungi ratio was significantly affected by the interaction of tillage and depth (p = 0.0029), and by the interaction of tillage, depth, and sampling time (p = 0.0608), with a lower ratio observed in the top 10 cm of MT compared to CT (Tables S2, S5).
Bacterial, crenarchaeal 16S rRNA, and fungal ITS gene copies in three soil layers (0–10, 10–20, and 30–60 cm) under minimum (MT) and conventional (CT) tillage from 2011 to 2020. The vertical line illustrates the year (2014) of the transition from conventional to organic farming. Data are shown as means ± standard errors (n = 4)
Discussion
Soil organic C contents affected by organic farming under non-inversion minimum tillage
In organic farming, adopting diverse crop rotations, mulching, and higher C inputs in the form of manure or slurry have been shown to increase in SOC in the topsoil, as demonstrated in a meta-analysis conducted by Gattinger et al. (2012). Their findings revealed higher SOC stocks in organic systems compared to conventional systems over a period of approximately 14 years. In our experiment, the SOC content under CT varied with time but, on average, remained relatively stable also during the 5-year period after the conversion to organic farming (Fig. 2). Similarly, the C stocks exhibited no significant changes during this period (Table 4). On the other hand, after only 5 years of conversion to organic farming, we observed significantly higher concentrations and stocks of SOC in the topsoil of MT, with an average increase of 12% in the top 20 cm (Fig. 2; Table 4). This increase in SOC stocks under MT can be attributed to both slower decomposition due to reduced tillage intensity and higher weed-derived C inputs in MT (Fig. 1; Table 1) (Krauss et al. 2017), as the organic fertilisation inputs were the same in both tillage systems (CT and MT).
Recent research indicates that labile DOC compounds from root exudates are efficiently anabolised into the microbially associated SOC pool (Kallenbach et al. 2016; Sokol et al. 2019). Moreover, the inputs of living roots can be up to 13 times more effective than litter inputs in increasing both the microbially associated SOC pool and the particulate organic C (POC) pool over several years (Sokol et al. 2019). However, it should be noted that the higher weed cover of MT, observed after the transition to organic farming, affected yields moderately (approximately 5% lower, on average) compared to CT over a 20-year period (Table 1).
Estimating how much C can be sequestered in the soil by agricultural measures, such as organic farming and non-inversion tillage practices, is essential for several European policies. Long-term organic reduced tillage trial conducted in Frick has shown a 25% increase in SOC in the top 10 cm after 15 years compared to conventional ploughing (Gadermaier et al. 2012; Krauss et al. 2020, 2022). In our study, SOC content in the top 10 cm of MT was 43% higher than in CT after 21 years, including 5 years of organic farming (Fig. 2); however, no differences were observed between tillage systems in deeper soil layers. Given that plant residues are incorporated into the topsoil layer (under CT in the top 25 cm and under MT in the top 10 soil layer), most changes in SOC are expected to occur in 0–20-cm soil depth as previously reported (Krauss et al. 2020, 2022). Over the entire 21-year period, MT accumulated 9.1 t/ha more SOC compared to CT in the 0–20-cm soil depth. This corresponds to an average annual accumulation rate of 0.43 t/ha, which is above the targets of the international “4 per 1000” initiative (https://4p1000.org/). In our soil, a 0.4% increase would correspond to 0.162 t SOC /ha per year. Notably, more than half of this significant difference in SOC accumulation occurred within only 5 years of adopting organic farming. During this period, MT accumulated 6.6 t SOC /ha more than CT, resulting in an annual 1.32-t/ha higher SOC accumulation rate. This rate is 3 times faster than the rate observed before the conversion to organic farming.
Whilst reduced tillage has been shown to have numerous positive effects on soil quality, as evidenced in our long-term experiment (Kaurin et al. 2015, 2018; Cania et al. 2020), ploughing still plays a crucial role in weed control, particularly in organic farming systems where herbicides are not used (Fig. 1). Combining no-till or minimum tillage with organic farming often leads to weed-related challenges, highlighting the need for alternative weed management strategies that do not rely on herbicides. It appears that weeds are important for soil quality indices, including the accumulation of SOC, so careful management and seeking for trade-offs between weed population and crop establishment need more attention in organic systems where reduced tillage is used. It is worth noting that weeds are not the sole solution; cover crops can offer similar C inputs whilst providing important environmental and agricultural services, including weed suppression.
Soil microbial biomass and composition of total microbial community during the transition to organic farming in dependence on the tillage system
It is well established in the literature that tillage affects soil physicochemical properties (e.g. aggregate stability, WHC, SOC, available C, nutrients, moisture, and temperature) (Haddaway et al. 2017; Bai et al. 2018; Torabian et al. 2019), which in turn significantly impact the living conditions for soil microbes (Young and Ritz 2000; Kuntz et al. 2013; Chen et al. 2020). Research conducted at our experimental site (e.g. Fig. 3; Kaurin et al. 2018; Cania et al. 2020) as well as studies by other authors (Li et al. 2018; Sauvadet et al. 2018; Krauss et al. 2020) consistently demonstrate a higher microbial biomass in the top layer of MT soils compared to conventional ploughing, regardless of whether the farming system is conventional or organic. This increase in microbial biomass can be attributed to a more favourable environment for microbes characterised by an increased availability of C, nutrients, and water in the top layer of MT compared to CT. Furthermore, it is noteworthy that in our study, microbial biomass has shown an overall increase following the conversion to organic farming practices under both tillage systems (Fig. 3). This enhancement of Cmic can be ascribed to greater C inputs in the form of manure, such as cattle slurry and compost, and the implementation of more diverse crop rotations, which provide higher quality organic inputs that support microbial growth (Bending et al. 2004; Six et al. 2006; Birkhofer et al. 2008). Our results are consistent with previous long-term studies, confirming that organic farming systems enhance soil microbial biomass (Mäder et al. 2002; Hartmann et al. 2006; Esperschütz et al. 2007). Significantly, our study reveals a more pronounced increase in microbial biomass in the topsoil under MT compared to CT, a trend visually illustrated in Fig. 3. This difference is further reflected by the significantly higher Cmic:SOC ratio observed throughout the entire transition period to organic farming (2015–2020) (Tables S5, S7). In the topsoil of MT, the Cmic:SOC ratio increased from 1.1% in 2015 to 2.5% in 2020, whereas in the topsoil of CT from 0.7% in 2015 to 1.9% in 2020. Importantly, the Cmic:SOC ratio in MT had already surpassed the proposed threshold value of 2.2% for soil in equilibrium between C mineralisation (respiration) and sequestration (Jenkinson and Ladd 1981; Anderson and Domsch 1989; Anderson 2003), which is consistent with the increased SOC stocks under MT (Fig. 2; Table 4). The rising Cmic:SOC ratio in the topsoil of CT following the adoption of organic farming practices suggests that even under CT conditions, SOC levels may begin to increase through the formation of microbial biomass and subsequent stabilisation of its necromass (Liang et al. 2019). A higher Cmic:SOC ratio indicates a better efficiency of microorganism in converting C sources into microbial biomass. Such efficiency is often associated with management practices promoting higher above-ground diversity (Araújo et al. 2008; Anderson and Domsch 2010; Li et al. 2020).
However, when examining microbial biomass below the top 10 cm, there were no significant differences between the two tillage systems in the 10–20-cm layer, whilst in the 30–60-cm soil layer, microbial biomass was higher under MT than under CT. The latter is a new finding for the subsoil that had not been previously reported by other authors. We hypothesise that this discrepancy may be attributed to the leaching of dissolved organic C (DOC) into deeper layers or the potential production of DOC by more abundant weed root exudates in MT at this soil depth (Fig. S1), leading to an increased microbial growth (Bradford et al. 2013; Sokol et al. 2019).
In addition to microbial biomass, higher SOC contents in the top 10 cm under MT increased the abundances of bacteria, archaea, and fungi (Fig. 4; Table S2), which is consistent with previous long-term studies conducted under various environmental conditions (Heinze et al. 2010; Li et al. 2018; Sauvadet et al. 2018). In our study, we observed that in the 5 years following the conversion to organic farming, the abundances of all three groups (bacteria, fungi, and archaea) increased in the top 10 cm under MT, whilst they remained relatively constant under CT. Similar results were reported by Krauss et al. (2020), who found increased concentrations of bacterial and fungal phospholipid fatty acids (PLFA) in the top 10 cm of soil under reduced tillage after 15 years of organic farming. Krauss et al. (2020) also observed the promotion of fungal community under MT, as indicated by a lower bacterial-to-fungi ratio. Although the bacterial-to-fungal ratio shift was less pronounced in our study, it was still lower under MT than CT, specifically in the top 10 cm of soil (Table S2). This shift towards a fungal community under MT was observed following the transition to the organic farming system (Table S2), suggesting that the increased C concentrations in the topsoil due to reduced tillage disturbance, coupled with organic farming practices, are driving the transition towards decomposition driven by fungi. Fungal hyphae are not disrupted by tillage, so they remain more intact at MT; this would also provide a greater opportunity for association with plant root rhizospheres (both crops and weeds). In particular, species that rely primarily on the presence of host plants are sensitive to the destruction of extraradical mycelia by frequent tillage (Orrù et al. 2021). A lower bacteria-to-fungi ratio may indicate a greater C sequestration in the soil, as fungi produce more biomass C per unit of metabolised C than bacteria, resulting in higher C immobilisation (Strickland and Rousk 2010; Yang et al. 2022). However, it is important to highlight that the method employed to assess bacterial and fungal communities differs between our and the studies mentioned above. We used the DNA-based qPCR approach to determine the abundance of 16S rRNA bacterial, crenarchaeal, and ITS fungal markers. In contrast, the results of the above studies were based on phospholipid fatty acid (PLFA) analysis. Despite the methodological differences, we can still draw comparable conclusions between studies.
Conclusions
Our long-term tillage experiment’s transition from conventional to organic management resulted in an increase in soil C stocks and microbial biomass, accompanied by a shift in microbial community composition in the top 10 cm of soil under the non-inversion minimum tillage (MT). Notably, SOC levels remained constant under conventional ploughing (CT) even during the 5 years following the conversion to organic farming, emphasizing the importance of implementing non-inversion reduced tillage practices for SOC accumulation. Whilst MT demonstrated benefits for soil quality compared to conventional ploughing, it is essential to acknowledge that weed cover and weed biomass were greater under MT. This factor potentially influenced the observed increase in SOC; however, our study did not fully elucidate this relationship, necessitating further investigation. Additionally, there is a pressing need for improving weed management strategies to encourage the adoption of reduced tillage practices in organic farming. Our findings highlight the synergistic effects of minimum tillage and organic farming on microbial communities. Over a 5-year period under organic management, we observed a notable increase in microbial biomass C under both tillage systems, which was further reflected in an increased Cmic:SOC ratio; however, these changes were particularly pronounced under the non-inversion minimum tillage. This suggests that the combination of minimum tillage and organic farming practices has a more significant positive impact on soil microbial communities than conventional ploughing in an organic farming context.
Data availability
Data available on request from the authors.
References
Anderson TH (2003) Microbial eco-physiological indicators to asses soil quality. Agric Ecosyst Environ 98:285–293. https://doi.org/10.1016/S0167-8809(03)00088-4
Anderson TH, Domsch KH (1989) Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol Biochem 21:471–479
Anderson TH, Domsch KH (2010) Soil microbial biomass: the eco-physiological approach. Soil Biol Biochem 42:2039–2043. https://doi.org/10.1016/j.soilbio.2010.06.026
Araújo ASF, Santos VB, Monteiro RTR (2008) Responses of soil microbial biomass and activity for practices of organic and conventional farming systems in Piauí state, Brazil. Eur J Soil Biol 44:225–230. https://doi.org/10.1016/j.ejsobi.2007.06.001
ARSO (2022) Arhiv meteoroloških podatkov. http://meteo.arso.gov.si/met/sl/archive/access
Bai Z, Caspari T, Ruiperez-Gonzalez M, Batjes NH, Mäder P, Bünemann EK, de Goede R, Brussaard L, Xu M, Santos Ferreira SS, Reintam E, Fan H, Mihelič R, Glavan M, Tóth Z (2018) Effects of agricultural management practices on soil quality: a review of long-term experiments for Europe and China. Agric Ecosyst Environ 265:1–7. https://doi.org/10.1016/j.agee.2018.05.028
Bending GD, Turner MK, Rayns F, Marx MC, Wood M (2004) Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol Biochem 36:1785–1792. https://doi.org/10.1016/j.soilbio.2004.04.035
Birkhofer K, Bezemer TM, Bloem J, Bonkowski M, Christensen S, Dubois D, Ekelund F, Fließbach A, Gunst L, Hedlund K, Mäder P, Mikola J, Robin C, Setälä H, Tatin-Froux F, Van der Putten WH, Scheu S (2008) Long-term organic farming fosters below and aboveground biota: implications for soil quality, biological control and productivity. Soil Biol Biochem 40:2297–2308. https://doi.org/10.1016/j.soilbio.2008.05.007
Bowles TM, Acosta-Martínez V, Calderón F, Jackson LE (2014) Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol Biochem 68:252–262. https://doi.org/10.1016/j.soilbio.2013.10.004
Bradford MA, Keiser AD, Davies CA, Mersmann CA, Strickland MS (2013) Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 113:271–281. https://doi.org/10.1007/s10533-012-9822-0
Braun-Blanquet J (1964) Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd edn. Springer Verlag, Berlin
Cania B, Vestergaard G, Suhadolc M, Mihelič R, Krauss M, Fliessbach A, Mäder P, Szumełda A, Schloter M, Schulz S (2020) Site-specific conditions change the response of bacterial producers of soil structure-stabilizing agents such as exopolysaccharides and lipopolysaccharides to tillage intensity. Front Microbiol 11:1–14. https://doi.org/10.3389/fmicb.2020.00568
Casagrande M, Peigné J, Payet V, Mäder P, Sans FX, Blanco-Moreno JM, Antichi D, Bàrberi P, Beeckman A, Bigongiali F, Cooper J, Dierauer H, Gascoyne K, Grosse M, Heß J, Kranzler A, Luik A, Peetsmann E, Surböck A et al (2016) Organic farmers’ motivations and challenges for adopting conservation agriculture in Europe. Org Agric 6:281–295. https://doi.org/10.1007/s13165-015-0136-0
Chen H, Dai Z, Veach AM, Zheng J, Xu J, Schadt CW (2020) Global meta-analyses show that conservation tillage practices promote soil fungal and bacterial biomass. Agric Ecosyst Environ 293:106841. https://doi.org/10.1016/j.agee.2020.106841
Egner MT, Riehm H, Domingo WR (1960) Untersuchungen uber die chemishe boden-analyse als grundlage fur die beurteilung des nahrsoffzustandes der boden. II. Chemiche extraktionsmethoden zur phosphor und kalimbestimmung kungl. K Lantbrukshoegskolans Ann 26:199–215
Esperschütz J, Gattinger A, Mäder P, Schloter M, Fließbach A (2007) Response of soil microbial biomass and community structures to conventional and organic farming systems under identical crop rotations. FEMS Microbiol Ecol 61:26–37. https://doi.org/10.1111/j.1574-6941.2007.00318.x
Fließbach A, Oberholzer HR, Gunst L, Mäder P (2007) Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric Ecosyst Environ 118:273–284. https://doi.org/10.1016/j.agee.2006.05.022
Gadermaier F, Berner A, Fliebach A, Friedel JK, Mäder P (2012) Impact of reduced tillage on soil organic carbon and nutrient budgets under organic farming. Renew Agric Food Syst 27:68–80. https://doi.org/10.1017/S1742170510000554
Gattinger A, Muller A, Haeni M, Skinner C, Fliessbach A, Buchmann N, Mader P, Stolze M, Smith P, Scialabba NE, Niggli U (2012) Enhanced top soil carbon stocks under organic farming. Proc Natl Acad Sci U S A 109:18226–18231. https://doi.org/10.1073/pnas.1209429109
Haddaway NR, Hedlund K, Jackson LE, Kätterer T, Lugato E, Thomsen IK, Jørgensen HB, Isberg PE (2017) How does tillage intensity affect soil organic carbon? A systematic review. Environ Evid 6:1–48. https://doi.org/10.1186/s13750-017-0108-9
Hartmann M, Fliessbach A, Oberholzer HR, Widmer F (2006) Ranking the magnitude of crop and farming system effects on soil microbial biomass and genetic structure of bacterial communities. FEMS Microbiol Ecol 57:378–388. https://doi.org/10.1111/j.1574-6941.2006.00132.x
Heinze S, Rauber R, Joergensen RG (2010) Influence of mouldboard plough and rotary harrow tillage on microbial biomass and nutrient stocks in two long-term experiments on loess derived Luvisols. Appl Soil Ecol 46:405–412. https://doi.org/10.1016/j.apsoil.2010.09.011
Hobbs PR, Sayre K, Gupta R (2008) The role of conservation agriculture in sustainable agriculture. Philos Trans R Soc B Biol Sci 363:543–555. https://doi.org/10.1098/rstb.2007.2169
Jenkinson DS, Ladd JN (1981) Microbial biomass in soil: measurement and turnover. In: Paul EA, Ladd JM (eds) Soil Biochemistry, vol 5. Marcel Decker, New York, pp 415–471
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31. https://doi.org/10.1016/0038-0717(95)00102-6
Joergensen RG, Brookes PC (2005) Quantification of soil microbial biomass by fumigation-extraction. In: Monitoring and Assessing Soil Bioremediation. Springer, Berlin, pp 281–295. https://doi.org/10.1007/3-540-28904-6_14
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630. https://doi.org/10.1038/ncomms13630
Kaurin A, Mihelič R, Kastelec D, Grčman H, Bru D, Philippot L, Suhadolc M (2018) Resilience of bacteria, archaea, fungi and N-cycling microbial guilds under plough and conservation tillage, to agricultural drought. Soil Biol Biochem 120:233–245. https://doi.org/10.1016/j.soilbio.2018.02.007
Kaurin A, Mihelič R, Kastelec D, Schloter M, Suhadolc M, Grčman H (2015) Consequences of minimum soil tillage on abiotic soil properties and composition of microbial communities in a shallow Cambisol originated from fluvioglacial deposits. Biol Fertil Soils 51:923–933. https://doi.org/10.1007/s00374-015-1037-9
Krauss M, Berner A, Perrochet F, Frei R, Niggli U, Mäder P (2020) Enhanced soil quality with reduced tillage and solid manures in organic farming — a synthesis of 15 years. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-61320-8
Krauss M, Ruser R, Müller T, Hansen S, Mäder P, Gattinger A (2017) Impact of reduced tillage on greenhouse gas emissions and soil carbon stocks in an organic grass-clover ley-winter wheat cropping sequence. Agric Ecosyst Environ 239:324–333
Krauss M, Wiesmeier M, Don A, Cuperus F, Gattinger A, Gruber S, Haagsma WK, Peigné J, Palazzoli MC, Schulz F, van der Heijden MG, Vincent-Caboud L, Wittwer RA, Zikeli S, Steffens M (2022) Reduced tillage in organic farming affects soil organic carbon stocks in temperate Europe. Soil Tillage Res 216:105262. https://doi.org/10.1016/j.still.2021.105262
Kuntz M, Berner A, Gattinger A, Scholberg JM, Mäder P, Pfiffner L (2013) Influence of reduced tillage on earthworm and microbial communities under organic arable farming. Pedobiologia 56:251–260. https://doi.org/10.1016/j.pedobi.2013.08.005
Lewis DB, Kaye JP, Jabbour R, Barbercheck ME (2011) Labile carbon and other soil quality indicators in two tillage systems during transition to organic agriculture. Renew Agric Food Syst 26:342–353. https://doi.org/10.1017/S1742170511000147
Li J, Shangguan Z, Deng L (2020) Dynamics of soil microbial metabolic activity during grassland succession after farmland abandonment. Geoderma 363:114167. https://doi.org/10.1016/j.geoderma.2019.114167
Li Y, Chang SX, Tian L, Zhang Q (2018) Conservation agriculture practices increase soil microbial biomass carbon and nitrogen in agricultural soils: a global meta-analysis. Soil Biol Biochem 121:50–58. https://doi.org/10.1016/j.soilbio.2018.02.024
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Chang Biol 25:3578–3590. https://doi.org/10.1111/gcb.14781
Loaiza Puerta V, Six J, Wittwer R, van der Heijden M, Pereira EIP (2019) Comparable bacterial-mediated nitrogen supply and losses under organic reduced tillage and conventional intensive tillage. Eur J Soil Biol 95:103121. https://doi.org/10.1016/j.ejsobi.2019.103121
Mäder P, Berner A (2012) Development of reduced tillage systems in organic farming in Europe. Renew Agric Food Syst 27:7–11. https://doi.org/10.1017/S1742170511000470
Mäder P, Fließbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697. https://doi.org/10.1126/science.1071148
Metzger MJ (2018) The environmental stratification of Europe [dataset]. University of Edinburgh. https://doi.org/10.7488/ds/2356
Montgomery DR (2007) Soil erosion and agricultural sustainability. Proc Natl Acad Sci U S A 104:13268–13272. https://doi.org/10.1073/pnas.0611508104
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700. https://doi.org/10.1128/aem.59.3.695-700.1993
Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C (2003) Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ Microbiol 5:787–797. https://doi.org/10.1046/j.1462-2920.2003.00476.x
Orrù L, Canfora L, Trinchera A, Migliore M, Pennelli B, Marcucci A, Farina R, Pinzari F (2021) How tillage and crop rotation change the distribution pattern of fungi. Front Microbiol 12:1–18. https://doi.org/10.3389/fmicb.2021.634325
Peigné J, Ball BC, Roger-Estrade J, David C (2007) Is conservation tillage suitable for organic farming? A review. Soil Use Manag 23:129–144. https://doi.org/10.1111/j.1475-2743.2006.00082.x
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/
Sans FX, Berner A, Armengot L, Mäder P (2011) Tillage effects on weed communities in an organic winter wheat-sunflower-spelt cropping sequence. Weed Res 51:413–421. https://doi.org/10.1111/j.1365-3180.2011.00859.x
Sauvadet M, Lashermes G, Alavoine G, Recous S, Chauvat M, Maron PA, Bertrand I (2018) High carbon use efficiency and low priming effect promote soil C stabilization under reduced tillage. Soil Biol Biochem 123:64–73. https://doi.org/10.1016/j.soilbio.2018.04.026
Six J, Elliott ET, Paustian K (1999) Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Sci Soc Am J 63:1350–1358
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569. https://doi.org/10.2136/sssaj2004.0347
Sokol NW, Kuebbing SE, Karlsen-Ayala E, Bradford MA (2019) Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol 221:233–246. https://doi.org/10.1111/nph.15361
Strickland MS, Rousk J (2010) Considering fungal: bacterial dominance in soils — methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395. https://doi.org/10.1016/j.soilbio.2010.05.007
Sun H, Koal P, Liu D, Gerl G, Schroll R, Gattinger A, Joergensen RG, Munch JC (2016) Soil microbial community and microbial residues respond positively to minimum tillage under organic farming in Southern Germany. Appl Soil Ecol 108:16–24. https://doi.org/10.1016/j.apsoil.2016.07.014
Torabian S, Farhangi-Abriz S, Denton MD (2019) Do tillage systems influence nitrogen fixation in legumes? A review. Soil Tillage Res 185:113–121. https://doi.org/10.1016/j.still.2018.09.006
Tuomisto HL, Hodge ID, Riordan P, Macdonald DW (2012) Does organic farming reduce environmental impacts? — A meta-analysis of European research. J Environ Manag 112:309–320. https://doi.org/10.1016/j.jenvman.2012.08.018
Watson CA, Atkinson D, Gosling P, Jackson LR, Rayns FW (2002) Managing soil fertility in organic farming systems. Soil Use Manag 18:239–247. https://doi.org/10.1079/sum2002131
White TJ, Bruns S, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Yang Y, Xie H, Mao Z, Bao X, He H, Zhang X, Liang C (2022) Fungi determine increased soil organic carbon more than bacteria through their necromass inputs in conservation tillage croplands. Soil Biol Biochem 167:108587. https://doi.org/10.1016/j.soilbio.2022.108587
Young IM, Ritz K (2000) Tillage, habitat space and function of soil microbes. Soil Tillage Res 53:201–213. https://doi.org/10.1016/S0167-1987(99)00106-3
Acknowledgements
Branko Majerič, owner of the experimental field, is sincerely acknowledged for his invaluable technical support, practical knowledge, and advice since the start of experiment. We thank the anonymous reviewers whose suggestions helped to strengthen this manuscript.
Funding
This study was financially supported by the CORE Organic Plus funding bodies within the FertilCrop project, by the Slovenian Research Agency (Ph.D. grant to Sara Pintarič, research projects CRP V4-2022 and L4-9315, and research program P4-0085), and by the Ministry of Agriculture, Forestry and Food (research project CRP V4-2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mihelič, R., Pintarič, S., Eler, K. et al. Effects of transitioning from conventional to organic farming on soil organic carbon and microbial community: a comparison of long-term non-inversion minimum tillage and conventional tillage. Biol Fertil Soils 60, 341–355 (2024). https://doi.org/10.1007/s00374-024-01796-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-024-01796-y