Abstract
Marked blood flow (BF) changes mediated by the autonomic neural and humoral systems may be important for orofacial hemodynamics and functions. However, it remains questionable whether differences in the autonomic vasomotor responses mediated by neural and humoral systems exist in the orofacial area. This study examined whether there are differences in changes in the BF and vascular conductance (VC) between the masseter muscle and lower lip mediated by autonomic neural and humoral systems in urethane-anesthetized rats. Electrical stimulation of the central cut end of the lingual nerve elicited BF increases in the masseter (mainly cholinergic) and lower lip (mainly non-cholinergic), accompanied by an increase in arterial blood pressure (ABP), while cervical sympathetic trunk stimulation consistently decreased BF at both sites. The lingual nerve stimulation induced a biphasic change in the VC in the masseter, consisting of an initial decrease and a successive increase. This decrease in VC was positively correlated with changes in ABP and diminished by guanethidine. Cervical vagus nerve stimulation also induced BF increases at both sites; the increases were greater in the masseter than in the lower lip. Adrenal nerve stimulation and isoproterenol administration induced BF increases in the masseter but not in the lower lip. These results indicate that cholinergic parasympathetic-mediated hemodynamics evoked by trigeminal somatosensory inputs are closely related to ABP changes. The sympathetic nervous system, including the sympathoadrenal system and visceral inputs, may be more involved in hemodynamics in the muscles than in epithelial tissues in the orofacial area.






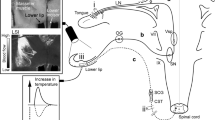
(Modified from Ishii et al. 2010). Photograph showing the stimulation site (arrowheads) of the left cervical vagus nerve in a supine rat. Scale bar represents 3 mm. B Typical examples of the effects of electrical stimulation of the left cervical vagus nerve for 20 s with 100 μA at 20 Hz using 2-ms pulses (CVN stim.) on the BF and VC in the masseter muscle and lower lip on the left side, and ABP. C Mean ± SEM of ΔBF and ΔVC in the masseter muscle (black bars) and lower lip (white bars) evoked by cervical vagus nerve stimulation (n = 5 in each group). The statistical significance of the differences from the base value without the stimulation was analyzed by paired t-test (*P < 0.05). A significant difference between data sets (P < 0.05) is indicated above the appropriate square bracket (unpaired t-test)



Similar content being viewed by others
References
Aijima R, Wang B, Takao T, Mihara H, Kashio M, Ohsaki Y, Zhang JQ, Mizuno A, Suzuki M, Yamashita Y, Masuko S, Goto M, Tominaga M, Kido MA (2015) The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J 29:182–192
Arbab MA, Wiklund L, Delgado T, Svendgaard NA (1988) Stellate ganglion innervation of the vertebro-basilar arterial system demonstrated in the rat with anterograde and retrograde WGA-HRP tracing. Brain Res 445:175–180
Berecek KH, Brody MJ (1982) Evidence for a neurotransmitter role for epinephrine derived from the adrenal medulla. Am J Physiol 242:H593–H601
Berthoud HR, Neuhuber WL (2000) Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85:1–17
Bruno RM, Ghiadoni L, Seravalle G, Dell’oro R, Taddei S, Grassi G (2012) Sympathetic regulation of vascular function in health and disease. Front Physiol 3:284
Burton AR, Fazalbhoy A, Macefield VG (2016) Sympathetic responses to noxious stimulation of muscle and skin. Front Neurol 7:109
Celander O (1954) The range of control exercised by the sympathico-adrenal system; a quantitative study on blood vessels and other smooth muscle effectors in the cat. Acta Physiol Scand 32:1–132
Delcanho RE (1995) Masticatory muscle pain: a review of clinical features, research findings and possible mechanisms. Aust Prosthodont J 9:49–59
Delcanho RE, Kim YJ, Clark GT (1996) Haemodynamic changes induced by submaximal isometric contraction in painful and non-painful human masseter using near-infra-red spectroscopy. Arch Oral Biol 41:585–596
Diana JN, Qian SF, Heesch CM, Barron KW, Chien CY (1990) Nicotine-induced skeletal muscle vasodilation is mediated by release of epinephrine from nerve terminals. Am J Physiol 259:H1718–H1729
Edvinsson L, McCulloch J, Uddman R (1982) Feline cerebral veins and arteries: comparison of autonomic innervation and vasomotor responses. J Physiol 325:161–173
Gibbins IL, Matthew SE, Bridgman N, Morris JL (1996) Sympathetic vasoconstrictor neurons projecting from the guinea-pig superior cervical ganglion to cutaneous or skeletal muscle vascular beds can be distinguished by soma size. Neurosci Lett 213:197–200
Greaves MW (1976) Physiology of skin. J Invest Dermatol 67:66–99
Guyton AC, Hall JE (1996) Textbook of medical physiology. 9th ed. W. B. saunders company
Handa Y, Caner H, Hayashi M, Tamamaki N, Nojyo Y (1990) The distribution pattern of the sympathetic nerve fibers to the cerebral arterial system in rat as revealed by anterograde labeling with WGA-HRP. Exp Brain Res 82:493–498
Hidaka O, Yanagi M, Takada K (2004) Mental stress-induced physiological changes in the human masseter muscle. J Dent Res 83:227–231
Holwerda SW, Restaino RM, Fadel PJ (2015) Adrenergic and non-adrenergic control of active skeletal muscle blood flow: implications for blood pressure regulation during exercise. Auton Neurosci 188:24–31
Ishii H, Niioka T, Izumi H (2007a) Parasympathetic vasodilator fibers in masseter muscle. J Oral Biosci 49:163–172
Ishii H, Niioka T, Izumi H (2009a) Circulating adrenaline released by sympathoadrenal activation elicits acute vasodilatation in the rat masseter muscle. Arch Oral Biol 54:486–494
Ishii H, Niioka T, Izumi H (2009b) Difference between male and female rats in cholinergic activity of parasympathetic vasodilatation in the masseter muscle. Arch Oral Biol 54:533–542
Ishii H, Niioka T, Izumi H (2010) Vagal visceral inputs to the nucleus of the solitary tract: involvement in a parasympathetic reflex vasodilator pathway in the rat masseter muscle. Brain Res 1312:41–53
Ishii H, Niioka T, Izumi H (2011) Parasympathetic reflex vasodilatation in the masseter muscle compensates for carotid hypoperfusion during the vagus-mediated depressor response. Brain Res 1370:145–153
Ishii H, Niioka T, Sudo E, Izumi H (2005) Evidence for parasympathetic vasodilator fibres in the rat masseter muscle. J Physiol 569:617–629
Ishii H, Niioka T, Watanabe H, Izumi H (2007b) Inhibitory effects of excess sympathetic activity on parasympathetic vasodilation in the rat masseter muscle. Am J Physiol Regul Integr Comp Physiol 293:R729–R736
Ishii H, Sato T (2017) Interactions between β-adrenergic vasodilation and cervical sympathetic nerves are mediated by α2-adrenoceptors in the rat masseter muscle. J Physiol Sci 67:699–709
Ishii H, Sato T, Izumi H (2014) Parasympathetic reflex vasodilation in the cerebral hemodynamics of rats. J Comp Physiol [b] 184:385–399
Izumi H (1999) Nervous control of blood flow in the orofacial region. Pharmacol Ther 81:141–161
Izumi H, Karita K (1992) Somatosensory stimulation causes autonomic vasodilatation in cat lip. J Physiol 450:191–202
Joyner MJ, Halliwill JR (2000) Sympathetic vasodilatation in human limbs. J Physiol 526:471–480
Kaji A, Shigematsu H, Fujita K, Maeda T, Watanabe S (1988) Parasympathetic innervation of cutaneous blood vessels by vasoactive intestinal polypeptide-immunoreactive and acetylcholinesterase-positive nerves: histochemical and experimental study on rat lower lip. Neuroscience 25:353–362
Kim HK, Kim KS, Kim ME (2013) Influence of test site and baseline temperature on orofacial thermal thresholds. J Orofac Pain 27:263–270
Kim YJ, Kuboki T, Tsukiyama Y, Koyano K, Clark GT (1999) Haemodynamic changes in human masseter and temporalis muscles induced by different levels of isometric contraction. Arch Oral Biol 44:641–650
Kulshreshtha P, Deepak KK (2013) Autonomic nervous system profile in fibromyalgia patients and its modulation by exercise: a mini review. Clin Physiol Funct Imaging 33:83–91
Kumakura K, Sato A, Suzuki H (1988) Direct recording of total catecholamine secretion from the adrenal gland in response to splanchnic nerve stimulation in rats. J Neurosci Methods 24:39–43
Kurosawa M, Sato A, Swenson RS, Takahashi Y (1986) Sympatho-adrenal medullary functions in response to intracerebroventricularly injected corticotropin-releasing factor in anesthetized rats. Brain Res 367:250–257
Lemon CH (2017) Modulation of taste processing by temperature. Am J Physiol Regul Integr Comp Physiol 313:R305–R321
Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W (2009) Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain 10:542–552
Maekawa K, Clark GT, Kuboki T (2002) Intramuscular hypoperfusion, adrenergic receptors, and chronic muscle pain. J Pain 3:251–260
Mito K, Sato T, Ishikawa R, Ramadhani R, Okada Y, Hirohata Y, Saito T, Ishii H (2021) Age-related decrease of cholinergic parasympathetic reflex vasodilation in the rat masseter muscle. Microvasc Res 104214
Niioka T, Ishii H, Izumi H (2009a) Involvement of vasoactive intestinal polypeptide in the parasympathetic vasodilatation of the rat masseter muscle. Arch Oral Biol 54:909–916
Niioka T, Ishii H, Izumi H (2009b) Regional differences in blood flow variation in rat masseter muscle. Arch Oral Biol 54:1022–1028
Ohke H, Sato T, Mito K, Terumitsu M, Ishii H (2020) Effect of the parasympathetic vasodilation on temperature regulation via trigeminal afferents in the orofacial area. J Physiol Sci 70:22
Sarelius I, Pohl U (2010) Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol (oxf) 199:349–365
Sato T, Ishii H (2015) Differences in control of parasympathetic vasodilation between submandibular and sublingual glands in the rat. Am J Physiol Regul Integr Comp Physiol 309:R1432–R1438
Sato T, Ishii H (2017) Regulation of hemodynamics in major salivary glands by parasympathetic vasodilation. J Oral Biosci 59:80–86
Sato T, Mito K, Ishii H (2020) Relationship between impaired parasympathetic vasodilation and hyposalivation in parotid glands associated with type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 318:R940–R949
Stern MD, Lappe DL, Bowen PD, Chimosky JE, Holloway GA Jr, Keiser HR, Bowman RL (1977) Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. Am J Physiol 232:H441–H448
Sudo E, Ishii H, Niioka T, Hirai T, Izumi H (2009) Parasympathetic vasodilator fibers in rat digastric muscle. Brain Res 1302:125–131
Suzuki N, Hardebo JE, Owman C (1990) Origins and pathways of choline acetyltransferase-positive parasympathetic nerve fibers to cerebral vessels in rat. J Cereb Blood Flow Metab 10:399–408
Szabo E, Csaki A, Boldogkoi Z, Toth Z, Koves K (2015) Identification of autonomic neuronal chains innervating gingiva and lip. Auton Neurosci 190:10–19
Vollmer RR, Balcita-Pedicino JJ, Debnam AJ, Edwards DJ (2000) Adrenal medullary catecholamine secretion patterns in rats evoked by reflex and direct neural stimulation. Clin Exp Hypertens 22:705–715
Watanabe H, Ishii H, Niioka T, Yamamuro M, Izumi H (2007) Occurrence of parasympathetic vasodilator fibers in the lower lip of the guinea-pig. J Comp Physiol [b] 178:297–305
Zempo H, Isobe M, Naito H (2017) Link between blood flow and muscle protein metabolism in elderly adults. J Phys Fitness Sports Med 6:25–31
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest, financial or otherwise, regarding this article.
Additional information
Communicated by G. Heldmaier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramadhani, R., Sato, T., Okada, Y. et al. Differences in the regulatory mechanism of blood flow in the orofacial area mediated by neural and humoral systems. J Comp Physiol B 193, 109–124 (2023). https://doi.org/10.1007/s00360-022-01470-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-022-01470-5