Abstract
To cope with periods of low food availability and unsuitable environmental conditions (e.g., short photoperiod or challenging weather), many heterothermic mammals can readily go into torpor to save energy. However, torpor also entails several potential costs, and quantitative energetics can, therefore, be influenced by the individual state, such as available energy reserves. We studied the thermal energetics of brown long-eared bats (Plecotus auritus) in the northern part of its distributional range, including torpor entry, thermoregulatory ability during torpor and how they responded metabolically to an increasing ambient temperature (Ta) during arousal from torpor. Torpor entry occurred later in bats with higher body mass (Mb). During torpor, only 10 out of 21 bats increased oxygen consumption (V̇O2) to a greater extent above the mean torpor metabolic rates (TMR) when exposed to low Ta. The slope of the torpid thermoregulatory curve was shallower than that of resting metabolic rate (RMR) during normothermic conditions, indicating a higher thermal insulation during torpor. During exposure to an increasing Ta, all bats increased metabolic rate exponentially, but the bats with higher Mb aroused at a lower Ta than those with lower Mb. In bats with low Mb, arousal was postponed to an Ta above the lower critical temperature of the thermoneutral zone. Our results demonstrate that physiological traits, which are often considered fixed, can be more flexible than previously assumed and vary with individual state. Thus, future studies of thermal physiology should to a greater extent take individual state-dependent effects into account.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many endothermic animals have a remarkable capacity to alter their metabolic rate (MR) and heart rate, and thereby body temperature (Schmidt-Nielsen 1997; Currie et al. 2014). By lowering their energy consumption to the bare minimum for survival, some heterothermic endotherms can reduce their MR by up to ~ 1000-fold compared to their active metabolism (Willis et al. 2005) when entering the energy saving state of torpor (Geiser 2021). The large potential energy savings gained from employing short-term torpor or hibernation during inclement conditions have been found to enhance individual survival probability and even reduce the risk of species extinctions (Geiser and Turbill 2009; Liow et al. 2009). Torpor is primarily present and most pronounced in small mammals such as rodents (Buck and Barnes 2000; Zervanos et al. 2010; Pretzlaff et al. 2021), marsupials (Franco et al. 2012), and bats (Dunbar and Tomasi 2006; Jonasson and Willis 2012; McGuire et al. 2021), as well as some birds (Wolf et al. 2020; Geiser 2021). As an adaptation to various conditions, the use of torpor is prevalent during both summer and winter, and in both the Southern and Northern hemispheres (Stawski et al. 2014; Ruf and Geiser 2015; Boyles et al. 2016; Geiser 2021; McGuire et al. 2021; Reher and Dausmann 2021).
For small mammals with a large surface area to volume ratio, obtaining enough energy to maintain a stable body temperature (Tb) is particularly challenging if they depend on a limited and seasonal food source (Buck and Barnes 2000), and employing torpor is a widely used strategy to cope with these challenges. At higher northern latitudes, the need for employing torpor on a day-to-day basis is further emphasized in nocturnal animals, as the length of summer night decreases with increasing latitude (Michaelsen et al. 2011). This means that nocturnal animals at high latitudes will need to build up sufficient energy reserves on a daily basis to compensate for the long days, and thereby long time between foraging bouts. Thus, the ability to enter torpor for shorter periods of time may be an essential strategy to both survive and reproduce during summers at high latitudes.
As torpor is not cost-free (Humphries et al. 2003; Boyles et al. 2020), there is a trade-off between the costs of maintaining torpor and the benefits of staying euthermic. Costs related to torpor include sleep deprivation (Humphries et al. 2003), memory loss (Millesi et al. 2001), and risk of predation (Estok et al. 2010; Haarsma and Kaal 2016), although the severity of the latter has been questioned (Turbill et al. 2011). In contrast, the benefits of maintaining a high Tb during daytime in summer include digestion of previously consumed food (Turbill et al. 2008) and allow for the development of fetus and lactation, which will be greatly delayed or reduced during torpor (Kurta et al. 1989; Dzal and Brigham 2013; Stawski et al. 2014).
It has recently been suggested that torpor is a more flexible trait than previously assumed (Reher et al. 2022). For example, torpor entry is often delayed (Matheson et al. 2010), and torpor duration decreased (Geiser and Broome 1993) in recently fed animals. Insectivorous bats (Chiroptera) at higher latitudes have a short reproductive season (Frafjord 2021), and rely on a food source which varies seasonally and occurs irregularly (Selås et al. 2013). Given their small body size, loss of heat is a major challenge when bats are faced with Tas below the thermoneutral zone (TNZ) (Bartels et al. 1998). Their limited potential for fat storage means that a substantial reduction of metabolic rate is the only possible option for non-migratory species when faced with longer periods of food shortage during winter at northern latitudes (Wermundsen and Siivonen 2010). Nevertheless, when and to which extent bats utilize torpor differs between species and environmental conditions (Stawski and Geiser 2010; Boyles et al. 2017), such as extreme heat (Reher and Dausmann 2021), unpredictable weather (Downs et al. 2012), or less suitable foraging conditions (Geiser et al. 2018).
As torpid bats may thermoregulate to some extent for short relocations within roost despite having a low Tb (Bartonička et al. 2017; Mayberry et al. 2017), it is reasonable to assume that there is an individual state component in studies investigating metabolic rate in bats that is often overlooked. Hence, as the cost of arousal increases with lower Ta (Wojciechowski et al. 2007), only bats with a higher energy reserve or better food availability can be expected to arouse at lower temperatures to counteract costs related to torpor (see Landes et al. 2020). When energy reserves are at a particularly low level, bats may not be able to arouse at low Ta as energetic reserves are insufficient to fuel arousal.
The brown long-eared bat (Plecotus auritus) is a medium sized (6–9 g) insectivorous Vespertilionid bat distributed widely across the Western Palearctic region (Wilson and Mittermeier 2019). Because the amount and availability of their prey is drastically reduced during the winter season, individuals must reduce their energy consumption to be able to hibernate through the winter. Towards the northern range of its distribution, P. auritus may also benefit from using torpor on a day-to-day basis throughout the year as night length, and thereby foraging opportunities, decrease with increasing latitude in summer (Michaelsen et al. 2011).
Some studies measuring metabolic rate have already been conducted within two populations of P. auritus in the western margin of its distribution range (Speakman et al. 1991; Webb et al. 1992; McLean and Speakman 2000; Becker et al. 2012, 2013). As P. auritus at high latitude are likely to enter short-term torpor on a day-to-day basis, understanding the cost and timing of arousal can improve our understanding of how bats survive in the northern hemisphere. Hence, in the present study, we experimentally studied this by inducing torpor in P. auritus and studied the physiology of torpor and the arousal from torpor. We predicted that individuals with more energy reserves should arouse earlier to reduce torpor-related costs, while those with low energy reserves would remain torpid for a longer period of time. We also predicted that P. auritus with larger energy reserves would utilize more energy to thermoregulate during torpor to reduce the costs and risks associated with deep torpor at low Ta.
Materials and methods
Bats exiting both potential and known roosts, or commuting along important flyways, were captured using mist-nets in Nittedal, Norway (60° 4′ 23″ N, 10° 52′ 20″ E) in June and July from 2019 to 2021. Upon capture, bats were immediately put into individual cloth bags, before measuring Mb to the nearest 0.1 g (Aweigh MB-50), forearm length to the nearest 0.1 mm (RS PRO 150 mm Digital Caliper 0.03 mm), while sex and reproductive state were determined. Mb was used as a proxy for energy reserves. Additionally, the wings of all captured bats were photographed using a standard DSLR camera with predetermined settings (1/160 s, f16, ISO 100), and an external flash providing back light to use the wing membrane for individual identification (see Amelon et al. 2017). Female bats that showed signs of reproduction (i.e., palpated abdomen or signs of lactation) were released after the individual morphometric measures. All males for which MR was measured were captured between June 1st and July 20th and did not show any signs of spermatogenesis.
After examination in the field, bats (N = 22) which were not reproductively active or not born the same year (i.e., closed epiphyseal gap) were brought back to an outdoor flight cage (2.5 m × 5 m × 2 m), which was equipped with bat boxes for roosting. Two of the four walls consisted of mesh netting, giving the cage an open air supply and a natural variation in light and environmental temperature. All bats were adult and consisted of 9 females and 13 males. On eight occasions, a single bat was brought back. Whereas, on some occasions, two (N = 5) or four (N = 1) bats were brought back at the same time. On one occasion, four bats were brought back in groups of two on two consecutive days. The following day, one bat was used for the experiment, while the other(s) were handfed Tenebrio molitor twice a day and given water ad libitum. Bats were always handfed before 21:00, so that they would be post-absorptive when the experiment started. When multiple bats were brought back the same day, females were always measured the following day, while males were measured on the 2nd day. Each bat was released at the capture site after sunset the same day the measurement had been performed. Thus, bats were held in captivity for 1 (N = 13), 2 (N = 5), 3 (N = 3) and 4 days (N = 1).
We measured metabolic rate indirectly as V̇O2 using open-flow respirometry. Bats were placed in a sealed chamber (325 ml) which was backlit from 03:30 to maintain a normal circadian rhythm, and placed inside a temperature-controlled cabinet. The closed chamber was connected to a pump (Eheim 100, EHEIM GmbH & Co., Deizisau, Germany), which supplied air from outside. Air was dried of humidity using Drierite before and after passing through the chambers, before finally entering a FOXBOX analyser (Sable Systems International, Las Vegas, NV, USA), which analyzed both V̇O2 and carbon dioxide production. The sample air also passed through an identical but empty chamber, which was used to perform baseline measurements for 15 min every hour (i.e., each set Ta consisted of 15 min of baseline measurements followed by 45 min of bat measurements). The analyser was zeroed at the onset of each field season using a 100% stock nitrogen. Additionally, the analyser was span-calibrated to 20.95% O2 in the middle of the first baseline at the onset of the experiment, as well as the prolonged baseline when Tset was reduced to 0 °C the following morning. Data recorded by the analyser were logged and stored in the software Expedata (Sable Systems International, Las Vegas, NV, USA) every 1 min.
To measure Ta, an iButton (model DS1923-F5, Dallas Semiconductor Inc., Dallas, TX, USA) was placed in the bottom of each chamber. The iButtons recorded temperature every minute to the nearest 0.001 °C. As other models of iButtons have been shown to emit ultrasound (Willis et al. 2009), we checked for this prior to the field season using a heterodyne bat detector (Model D200, Petterson Elektronik AB, Uppsala, Sweden) and observed no indication of ultrasound noise. Additionally, iButtons were calibrated in a water bath against a precision thermometer and revealed little difference between iButtons, similar to what was reported by Davidson et al. (2003). The upper half of the chamber was covered with mesh netting, on which the bat could roost. When placed inside the chamber, the cabinet was set to 5 °C to motivate the bat to enter torpor. Bats were placed in the chamber at differing times throughout the night (mean ± SD, 160.5 ± 70.3 min after sunset). Upon arrival to the flight cage, one bat was immediately placed into the chamber and thus not handfed or supplied with water before the experiment. But digestive state presumably differed as time of capture differed between bats. At approximately 09:00, the following morning, the set temperature (Tset) was reduced to 0 °C. Thereafter, the Tset was increased by 5 °C every hour until it reached 25 °C, after which we increased it by 3 °C per h until it reached 37 °C. For one measurement, technical issues in the recording computer meant we could only record data at Ta > 26 °C for one bat. The lowest mean Ta at which MR was measured was 0.99 ± 1.28 °C (± SD, N = 21). The highest mean Ta was 36.14 ± 1.40 °C (± SD, N = 22). The total length of each experiment lasted on average for 16.0 ± 1.4 h (± SD, N = 22).
Incurrent flow rate was set at 315 mL min−1 when the bat was placed in the chamber. At approximately 09:00, flow rate was reduced, as all bats were in torpor, and kept between 101 and 248 mL min−1 while the bat was torpid. To determine when a bat had exited torpor, we routinely observed the O2 measurements in the software Expedata, as well as observing via a camera in the temperature-controlled cabinet. As soon as the bat exited torpor, flow rate was increased to 315 mL min−1.
To calculate V̇O2, we selected a series of stable values over at least five consecutive measurements (i.e., at least 5 min) at each Tset. Using the software R (version 4.0.2), the lowest 5 min mean within this selection was extracted using the runMean() function in the TTR package (Ulrich 2021) for further analysis. Overall, the Ta generally fluctuated slightly within each hour. At Tset of 0 °C, the Ta fluctuated above the Tset, whereas between Tset of 5 and 37 °C the Ta fluctuated slightly below the Tset. At the lowest Tset, we selected at least five consecutive measurements when the Ta was lowest to calculate to which extent the bats showed active thermoregulation (Table S1).
We corrected for drift and calculated V̇O2 using Eq. (10.5) in Lighton (2018),
where FRi is the incurrent air flow rate, FiO2 is the fractional content of incurrent oxygen, FeO2 is the fractional content of excurrent oxygen, FiCO2 is the fractional content of incurrent carbon dioxide, while FeCO2 is the fractional content of excurrent carbon dioxide.
Bats were weighed (± 0.1 g) immediately before being placed in the chamber, and again immediately after the metabolic trial. As V̇O2 is very low during torpor, mass loss is correspondingly very low during torpor. Therefore, to be able to calculate a more precise value of mass-specific MR, we calculated body mass at any given time based on the equation:
where Mcur is the calculated mass at each minute, Mprev is the calculated mass in the previous minute, V̇O2cur is the O2 consumption in the current minute, V̇O2tot is the total calculated O2 consumption over the entire experiment, and Mt is the total loss of Mb over the entire experiment. During baseline and periods where the flow rate was too high and the oxygen analyzer therefore had problems picking up the minor V̇O2 of torpid bats, we calculated the mean V̇O2 of the 5 min before and after this period and assigned it to these periods as the V̇O2 for use in the calculations of Mcur.
As we investigated the V̇O2 measurements of each individual visually, it was evident that 45% of the bats (N = 10) maintained torpor at higher temperatures (i.e., > 30 °C), as MR increased exponentially with increasing Ta. These were considered thermoconforming within the TNZ (referred to as Group 1). The remaining bats (N = 12) aroused at lower Ta. The latter group was defined as euthermic for the rest of their experiment as they did not reenter torpor at higher Ta (referred to as Group 2). All stable measurements at a lower Ta before arousal were considered TMR. As previous studies have shown that bats thermoregulate below 1.8–6.7 °C (Willis et al. 2005; Stawski and Geiser 2011; Currie et al. 2018), an increase in V̇O2 consumption with decreasing Ta at low ambient temperatures was defined as active thermoregulation, as they actively increased heat production.
We chose to analyse these two groups separately, as the latter group showed more stable readings over multiple temperatures within the TNZ. To quantify the arousal events, we defined an arousal as the point in time when V̇O2 in torpid bats increased substantially. The Ta measured at the same minute as metabolic rate started to increase was used as the Ta of the start of the arousal. The length of the arousal in minutes was quantified from the start of the increase to the time at which mass-specific V̇O2 peaked or stabilized. Although bats are known to overshoot their V̇O2 relative to the RMR during arousals at the same Ta (Turbill et al. 2008), the decrease phase of the arousal was not included in the analyses as it was often interrupted by baseline measurements. We used the difference in V̇O2 between the minute at which the V̇O2 peaked and the last measurement before the increase in V̇O2 started as a proxy for V̇O2 during the arousal (Figures S1 and S2).
Statistical analyses
Body mass
All analyses were performed using R (version 4.0.2). Results are presented in the language of evidence as suggested by Muff et al. (2022).
To analyze factors influencing changes in Mb upon capture we fitted a linear model with Mb as a response variable, and days after June 1st, time after sunset (minutes), and sex as fixed effects, assuming a Gaussian error distribution. Two males were measured two times in different years, but since the recaptures were a year apart, and Mb differed highly between captures for both individuals, we treated the measurements as independent. To see if there were any differences in Mb between the bats which did not exit torpor prior to the TNZ (Group 1) and those that did (Group 2), we performed linear regression analyses with Mb at the start of the experiment as a response variable, and group as a fixed effect. Additionally, to investigate if Mb at the onset of the experiment affected the mass loss and the Mb at the end of the experiment, we fitted simple linear regressions with Mb at the end of the experiment as the response variable, and Mb at the onset of the experiment and Group as fixed effects.
Metabolic rate
As the timing of torpor entry differed between bats, we fitted a linear regression model using the lm() function with time spent before torpor entry in minutes as the response variable, and Mb at the onset of the experiment as the explanatory variable. To estimate how metabolic rate differs with increasing Ta in the bats which aroused at lower Ta, we fitted a linear regression model using the lm() function with V̇O2 as a response variable, and Ta as an explanatory variable. Thereafter, we used Davies test, using the davies.test() function in the segmented package (Muggeo 2008) to check for the presence of a significant inflection point in the relationship between V̇O2 and Ta. If an inflection point was identified, we performed a broken stick regression using the segmented() function in the segmented package (Muggeo 2008) to identify at which Ta the relationship with V̇O2 changed. To check if the segmented() function provided a better fit than the initial lm() function, we performed ANOVA analysis on both functions. After identifying a potential inflection point, we defined all measurements at Ta below the inflection point as RMR and all measurements at Ta above the inflection point as BMR.
The RMR was estimated using a linear mixed-effects model using the lmer() function (Bates et al. 2015) with V̇O2 below the inflection point as a response variable, and Ta and Mb as a fixed effects. Individual bat ID was added as a random effect. A similar analysis was performed on BMR with V̇O2 above the inflection point as a response variable. We fitted an exponential growth curve to the TMR data for each group using the nls() function in R and performed a linear mixed model with minimum TMR of each individual as the response variable and days after June 1st, sex, and group as fixed effects.
Active thermoregulation
To estimate the slope of active thermoregulation during torpor at low Ta, we performed a linear mixed-effects model with V̇O2 as the response variable, and Ta at which the measurement was taken as a fixed effect. Individual ID was added as a random effect. This analysis consisted of a subset of individuals, as some individuals did not show any clear increase in V̇O2 at low Ta (N = 8), while in some cases the difference in Ta between two means were too big to give a reliable estimate (N = 3).
Arousal
To better understand the physiology and timing of arousals, we performed four separate analyses. First, we performed a simple t test to compare the V̇O2 during arousal between the two groups. Second, to estimate arousal costs with decreasing Ta, we fitted a simple linear regression with V̇O2 during arousal as the response variable, and Ta as an explanatory variable. Third, we performed a simple t test to compare the number of minutes needed to arouse between the two groups. Fourth, we fitted a simple linear regression with the Ta at which arousal occurred as a response variable and Mb at the onset of the experiment as an explanatory variable to investigate if body condition affected arousal. This was also repeated with estimated Mb at the timing of arousal as an explanatory variable. We performed simple linear regressions as all bats in our study only aroused once per experimental run, and only completed one torpor bout.
Results
Body mass
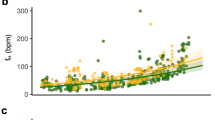
Average Mb upon capture was 7.82 ± 0.96 g (range 6.6–10.7 g, N = 22), while forearm length averaged 39.4 ± 1.3 mm (range 36.7–43.2 mm). There was strong evidence that Mb increased with time after sunset (Mb 0.009 g min−1 ± SE 0.003, p = 0.004), but no evidence that it increased with days after June 1st (p = 0.86) or forearm length (p = 0.40), and there was no difference between sexes (p = 0.48). In contrast, the average Mb for all P. auritus (N = 90, n = 156) captured in the study area between 2017 and 2021 was 8.30 ± 1.31 g (N = 156), which is higher than for the bats included in the present study (t33.3 = − 2.08, p = 0.045). In addition to a higher Mb with time after sunset, there was also very strong evidence that Mb was positively related to forearm length, and that there was a difference between sexes (Table 1, Fig. 1). Because females have an earlier reproductive period and had greater Mb than males (females: 8.62 ± 1.32 g, N = 112; males: 7.49 ± 0.91 g, N = 44, t113.4 = 6.1, p < 0.0001), and as females had longer forearms (females: 39.6 ± 1.2 mm, N = 112; males: 38.9 ± 1.4 mm, N = 44, t64.0 = 3.2, p = 0.002) we fitted linear mixed-effects models for each sex separately (Table 1).
Fixed effects influencing body mass (g) in captured P. auritus (N = 89, n = 157) in Nittedal, Norway between 2017 and 2021. Body mass increased with a forearm length (mm), b time after sunset (min), and c was higher in females than in males. In a and b solid lines show estimated relationships and shaded polygons show 95% confidence limits. In c the black dots show the estimated average and the whiskers show the 95% CIs. Dots show measured values
At the onset of the experiment, there was strong evidence that average Mb for the bats that aroused below Tlc (8.29 ± 1.04 g, N = 12, Group 2) was higher (p = 0.009) than for the bats that aroused above Tlc (7.25 ± 0.53 g, N = 10, Group 1, Fig. 2a). There was strong evidence that Mb at the end of the experiment increased with higher Mb at the onset of the experiment (g = 1.73 + 0.67 × g, r2 = 0.87, p < 0.005, Fig. 2b), but did not differ between the bats which aroused from torpor prior to entering the TNZ and those that did not (p = 0.88). Mb showed a noticeable decrease at the onset of the experiment, followed by a long period in which the Mb remained stable during torpor, until it decreased gradually at higher Ta (Figures S1 and S2).
Body mass (g) at a the onset of the experiment was higher in bats that exited torpor before the estimated lower critical temperature (Tlc) of the TNZ (Group 2), than those that did not (Group 1). Body mass (g) at b the end of the experiment was higher in bats with a higher body mass at the onset of the experiment (g = 1.73 + 0.67 × g, r2 = 0.87, p < 0.005)
Metabolic rate
Bats entered torpor after 102.8 ± 85.3 min (± SD, N = 19) into the experiment. Torpor entry occurred later in bats that weighed more at the onset of the experiment (minutes = − 284.2 + 51.5 × g, r2 = 0.36, p = 0.005, N = 19, Fig. 3). Broken stick regression revealed that V̇O2 decreased until 29.7 °C and provided a better fit than the linear model (F = 42.5 vs F = 23.2, respectively). RMR increased with decreasing temperature (mL O2 h−1: 119.1 − 2.94 × Ta, p = 0.002, N = 8, n = 12), but was not affected by Mb (p = 0.658). We, therefore, removed Mb from the model and repeated the analysis (mL O2 h−1: 104.9 − 2.9 × Ta, p = 0.002, N = 8, n = 12, Fig. 4a). BMR was affected by neither Mb (p = 0.403), nor Ta (p = 0.549), and was estimated at 15.5 ± 3.4 mL O2 h−1 (N = 11, n = 24). TNZ was between 29.7 and at least up to 36.4 °C. Four individual bats measured had an increased MR at 36.4 ± 0.6 °C (range 35.7–37.0 °C).
Metabolic rate (mL O2 h−1) as a function of ambient temperature (Ta °C). Metabolic rate of bats which a exited torpor before the TNZ, and bats which b remained torpid at temperatures above Tlc. The dotted line in a shows the increase in metabolism with decreasing Ta (2.91 mL O2 h−1 °C−1, N = 8, n = 12) for euthermic bats (RMR). While the dotted line present in both a and b at Ta below 6.73 °C shows the increase in metabolism with decreasing Ta (1.30 mL O2 h−1 °C−1, N = 10, n = 21). Blue circles show measurements of included in broken stick regression for RMR and BMR (15.5 ± 3.4 mL O2 h−1, N = 11, n = 24), indicated by the black line which also shows the range of the TNZ (29.67–36.4 °C). Black circles in both plots show measurements of TMR. Blue line in both plots indicate the exponential growth curve of TMR with increasing Ta (a), 0.127 × 1.148Ta and b 0.141 × 1.151Ta. Red triangles show plots where bats showed active thermoregulation (colour figure online)
During torpor, V̇O2 increased exponentially with increasing temperatures for bats that exited torpor before the TNZ was reached (mL O2 h−1 = 0.127 × 1.148Ta, r2 = 0.65, N = 12, n = 43, Fig. 4a). A similar relationship was found for bats that remained torpid within the TNZ (mL O2 h−1 = 0.141 × 1.151Ta, r2 = 0.86, N = 10, n = 86, Fig. 4b). The minimum measured TMR (0.644 ± 0.493 O2 h−1, N = 21) occurred at 10.1 ± 5.2 °C (N = 21) but varied between individuals (range 0.3–18.4 °C). The minimum measured TMR per individual was not related to days after June 1st, nor did it differ between bats that aroused before the Tlc and those that did not, or between males and females (p = 0.756).
Active thermoregulation during torpor
When exposed to Tas below 6.7 °C, as indicated by the intercept of the thermoregulatory curve of thermoregulating bats and the exponential curve of TMR, bats which showed active thermoregulation increased TMR by 1.30 mL O2 h−1 °C−1 (N = 10, n = 21; Fig. 4). Average Mb of the bats that increased V̇O2 at low Ta was marginally higher (8.34 ± 1.06 g, N = 10) at the onset of the experiment then the average Mb of those that did not increase V̇O2 (7.46 ± 0.81, N = 8, t16.0 = 1.99, p = 0.064).
Arousal
Bats that exited torpor below the Tlc aroused at 22.9 ± 3.5 °C (N = 11), while bats that remained torpid into the TNZ aroused at 31.9 ± 3.7 °C (N = 7). In four cases, arousal was either not apparent, or occurred during baseline measurements. There was strong evidence for a higher V̇O2 during arousal (t11.24 = 3.96, p = 0.002) in the bats that aroused at a lower temperature (73.78 ± 40.99 mL O2 h−1), compared to those that aroused later (23.44 ± 7.97 mL O2 h−1), and increased with decreasing Ta (mL O2 h−1 = 198.38 − 5.46 × Ta, p < 0.001). The number of minutes the bats needed to arouse did not differ between the bats which aroused at Ta < 29.7 °C, and those that aroused at Ta > 29.7 °C (t11.09 = − 1.39, p = 0.19). Bats with higher Mb at the onset of the experiment aroused at lower ambient temperatures (Ta = 54.38 − 3.56 × g, p = 0.003, Fig. 5b), but also had a higher estimated Mb when arousal occurred (Ta = 58.08 − 4.42 × g, p = 0.004), despite having delayed torpor entry for longer at the onset of the experiment.
Discussion
By studying how P. auritus close to the northern range of its distribution respond physiologically to different temperature conditions, we found that individual state influenced quantitative energetics in this species. As predicted, individual bats with a higher Mb aroused from torpor at lower Ta, while individuals with a lower Mb postponed arousal to higher Ta. Although we observed substantial active thermoregulation at low temperatures in almost half of the measured bats, the relationship between increased V̇O2 at low Ta and Mb was unclear, with only a marginal difference in Mb.
Mb at time of capture increased as expected with time after sunset. As bats at high latitudes are subject to short nights for foraging during summer (Frafjord 2013; Michaelsen et al. 2018), they need to build up a large energy reserve on an almost daily basis to survive and reproduce. At the same time, they stay in the roost for approximately 20 h each day. Thus, depending on the amount of time during the day spent torpid, they generally leave the roost with a relatively low Mb as they will have consumed a large portion of their gut fill.
TMR was similar between bats that aroused before the Tlc, and those that did not. TMR increased exponentially with increasing Ta, and is similar to that of equally sized vespertilionid bats in the southern hemisphere (Geiser and Brigham 2000; Turbill et al. 2008; Stawski and Geiser 2011). Thus, further supporting the notion that torpor in subtropical vespertilionid bats does not differ from temperate vespertilionid bats (Stawski and Geiser 2011; Fjelldal et al. 2022), as torpor is a mechanism to cope with energy limitation at low Ta regardless of the habitat.
Interestingly, only half of the bats in the present study showed signs of active thermoregulation during torpor at low Ta, despite all 21 bats being exposed to Ta below the estimated critical Ta of 6.7 °C. However, bats which showed active thermoregulation only had a marginally higher Mb at the onset of the experiment compared to those that did not. In a study conducted by Currie et al. (2018) where both RMR and the torpor thermoregulation curve of bats was estimated, the metabolic rate of both curves had an equal increase. A trend which is similar to what is found in other animals that employ torpor (see Geiser 2021). In contrast, in our study, active thermoregulation at low Ta was with a much shallower increase in TMR than what was observed in resting bats (i.e., RMR).
In the literature, the critical Ta is often referred to as a species or population specific temperature threshold, which bats have evolved as long-term adaptations to their respective environmental conditions (Stawski and Geiser 2011). However, as the critical Ta estimated in the present study is higher than the Ta in which P. auritus regularly hibernate at this latitude (Wermundsen and Siivonen 2010), and considerably higher than the lowest measured Tb of − 2 °C (Eisentraut 1956), this indicates that individual bats may choose the level of thermoregulation to some extent at different Ta to avoid torpor-related costs. A lower increase in V̇O2 when thermoregulating at low Ta may be related to a higher level of thermal insulation. As the length of fur coat is equal during the individual experiments here, the assumed difference in insulation could be due to a greater peripheral part of the body being kept cold, and Tb is only defended in vital parts of the body. Due to this increase in insulation, bats will only need to increase V̇O2 to a lesser extent to prevent tissue damage to vital organs. It is also worth noting that our measurements were performed during summer, as bats may defend a higher Tb at this time of year as opposed to during the hibernation season. But the question as to why some bats choose to thermoregulate and some do not, remains unanswered.
It is becoming more and more clear that Mb not only affects metabolism but can also affect the metabolic strategy of the animal. As observed here, bats with higher Mb readily spent more energy to delay torpor. All bats in our study showed similar TMR when thermoconforming but differed in level of exploration at the onset of the experiment, active thermoregulation at lower Ta, and timing of arousal. Bats with a higher Mb at the onset of the experiment aroused at lower Ta, and lost more Mb during the experiment. This loss in Mb was also related to these bats delaying torpor for a longer period at the onset of the experiment. Similarly, recently fed Myotis lucifugus delay torpor entry independent of temperature (Matheson et al. 2010). Similar behavioural responses have been seen in edible dormice (Glis glis), where heavier animals aroused more frequently and stayed euthermic for longer (Bieber et al. 2014), and woodchucks (Marmota monax), which defended a higher Tb when more energy was available (Zervanos et al. 2013).
The delay of torpor in recently fed bats, along with an earlier arousal, is presumably related to a trade-off between different costs and benefits of torpor. Although there are potential costs of maintaining torpor for longer time periods, such as sleep deprivation (Humphries et al. 2003), predation (Estok et al. 2010; Haarsma and Kaal 2016), and buildup of waste materials (Thomas and Geiser 1997; Ben-Hamo et al. 2013; Landes et al. 2020), the physiological benefits include water and energy conservation. Thus, on a day-to-day basis during the active season of the year, the physiological benefits of limiting energy consumption and water loss is likely to outweigh the costs.
As bats in our study area, and at similar latitudes, will daily spend up to 20 h in roosts without access to water (i.e., barns or trees), the risk of dehydration is potentially a contributing reason to remain in torpor during daytime. As water consumption prior to the experiment was not controlled for, Mb could also be affected by hydration level in individual bats. This effect may be exacerbated in typical studies of metabolic rate, as dry air will lead to an increased evaporative water loss.
Another potential explanation for the earlier arousal can be that heavier bats exert the reserve energy to allow for restorative activities, such as sleep (Humphries et al. 2003), protein synthesis (Heldmaier et al. 2004; Landes et al. 2020), or even digestion of previously consumed food (Turbill et al. 2008). As all bats left fecal droppings in the chamber during the experiment, some digestion must have occurred. It is, however, unknown whether this occurred prior to torpor entry, following arousal, or both. Thus, reinitiating digestion and being able to move away from potential threats in an unfamiliar environment may explain why bats with larger Mb opted for leaving torpor at an earlier stage. Additionally, in a wider context, it may be beneficial for individual bats to arouse earlier to allow for social interactions, such as grooming (Chaverri et al. 2018) and information transfer (Gager 2018). Thus, the optimal timing of arousal from torpor is probably a trade-off between the physiological benefits of torpor and the ecological costs of missing out on the benefits of euthermia. Accordingly, the condition of an individual bat should affect its decision-making under these opposing pressures.
In summary, the present study highlights how metabolism of individual bats is affected by their condition, as bats with a larger energy reserve readily arouse at lower Ta, possibly to counteract the negative effects of torpor. Similarly, active thermoregulation occurred in only half of all measured bats and at Tas higher than what they normally experience during hibernation. Additionally, the increase in metabolic rate during active thermoregulation when torpid has a shallower slope compared to that of normothermic bats resting at similar temperatures below the TNZ. This indicates that the bats altered their thermal conductance by an increased insulation. Essentially, when and to which extent individual bats actively increase heat production may be behaviorally flexible, and not physiologically fixed.
Data availability
The data collected and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- T b :
-
Body temperature
- T a :
-
Ambient temperature
- TMR:
-
Torpid metabolic rate
- TNZ:
-
Thermoneutral zone
- M b :
-
Body mass
- V̇O2 :
-
Oxygen consumption
- T set :
-
Set temperature
- BMR:
-
Basal metabolic rate
- RMR:
-
Resting metabolic rate
- T lc :
-
Lower critical temperature
References
Amelon SK, Hooper SE, Womack KM (2017) Bat wing biometrics: using collagen-elastin bundles in bat wings as a unique individual identifier. J Mammal 98:744–751. https://doi.org/10.1093/jmammal/gyx018
Bartels W, Law BS, Geiser F (1998) Daily torpor and energetics in a tropical mammal, the northern blossom-bat Macroglossus minimus (Megachiroptera). J Comp Physiol B 168:233–239. https://doi.org/10.1007/s003600050141
Bartonička T, Bandouchova H, Berková H, Blažek J, Lučan R, Horáček I, Martínkova N, Pikula J, Řehák Z, Zukal J (2017) Deeply torpid bats can change position without elevation of body temperature. J Therm Biol 63:119–123. https://doi.org/10.1016/j.jtherbio.2016.12.005
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Becker NI, Encarnação JA, Kalko EKV, Tschapka M (2012) The effects of reproductive state on digestive efficiency in three sympatric bat species of the same guild. Comp Biochem Physiol A 162:386–390. https://doi.org/10.1016/j.cbpa.2012.04.021
Becker NI, Encarnação JA, Tschapka M, Kalko EKV (2013) Energetics and life-history of bats in comparison to small mammals. Ecol Res 28:249–258. https://doi.org/10.1007/s11284-012-1010-0
Ben-Hamo M, Muñoz-Garcia A, Willians JB, Korine C, Pinshow B (2013) Waking to drink: rates of evaporative water loss determine arousal frequency in hibernating bats. J Exp Biol 216:573–577. https://doi.org/10.1242/jeb.078790
Bieber C, Lebl K, Stalder G, Geiser F, Ruf T (2014) Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct Ecol 28:167–177. https://doi.org/10.1111/1365-2435.12173
Boyles JG, McGuire LP, Boyles E, Reimer JP, Brooks CAC, Rutherford RW, Rutherford TA, Whitaker JO, McCracken GF (2016) Physiological and behavioral adaptations in bats living at high latitudes. Physiol Behav 165:322–327. https://doi.org/10.1016/j.physbeh.2016.08.016
Boyles JG, Boyles E, Dunlap RK, Johnson SA, Brack V Jr (2017) Long-term microclimate measurements add further evidence that there is no “optimal” temperature for bat hibernation. Mamm Biol 86:9–16. https://doi.org/10.1016/j.mambio.2017.03.003
Boyles JG, Johnson JS, Blomberg A, Lilley TM (2020) Optimal hibernation theory. Mamm Rev 50:91–100. https://doi.org/10.1111/mam.12181
Buck CL, Barnes BM (2000) Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regulat Integr Comp Physiol 279:R255–R262. https://doi.org/10.1152/ajpregu.2000.279.1.R255
Chaverri G, Ancillotto L, Russo D (2018) Social communication in bats. Biol Rev 93:1938–1954. https://doi.org/10.1111/brv.12427
Currie SE, Körtner G, Geiser F (2014) Heart rate as a predictor of metabolic rate in heterothermic bats. J Exp Biol 217:1519–1524. https://doi.org/10.1242/jeb.098970
Currie SE, Stawski C, Geiser F (2018) Cold-hearted bats: uncoupling of heart rate and metabolism during torpor at sub-zero temperatures. J Exp Biol 221:170894. https://doi.org/10.1242/jeb.170894
Davidsson AJ, Aujard F, London B, Menaker M, Block GD (2003) Thermochron iButtons: an inexpensive method for long-term recording of core body temperature in untethered animals. J Biol Rhythms 18:430–432. https://doi.org/10.1177/0748730403256066
Downs CT, Zungu MM, Brown M (2012) Seasonal effects on thermoregulatory abilities of the Wahlberg’s epauletted fruit bat (Epomorphus wahlbergi) in KwaZulu-Natal, South Africa. J Therm Biol 37:144–150. https://doi.org/10.1016/j.jtherbio.2011.12.003
Dunbar MB, Tomasi TE (2006) Arousal patterns, metabolic rate, and an energy budget of eastern red bats (Lasiurus borealis) in winter. J Mammal 87:1096–1102. https://doi.org/10.1644/05-MAMM-A-254R3.1
Dzal YA, Brigham RM (2013) The tradeoff between torpor use and reproduction in little brown bats (Myotis lucifugus). J Comp Physiol B 183:279–288. https://doi.org/10.1007/s00360-012-0705-4
Eisentraut M (1956) Der Winterschlaf mit seinen ökologischen und physiologischen Begleiterscheinungen. G Fischer, Jena
Estók P, Zsebók S, Siemers BM (2010) Great tits search for, capture, kill and eat hibernating bats. Biol Lett 6:59–62. https://doi.org/10.1098/rsbl.2009.0611
Fjelldal MA, Sørås R, Stawski C (2022) Universality of torpor expression in bats. Physiol Biochem Zool. https://doi.org/10.1086/720273
Frafjord K (2013) Influence of night length on home range size in the northern bat Eptesicus nilssonii. Mamm Bio 78:205–211. https://doi.org/10.1016/j.mambio.2012.06.005
Frafjord K (2021) Activity patterns of the soprano pipistrelle Pipistrellus pygmaeus throughout the year in southern Norway. BMC Zool 6:1. https://doi.org/10.1186/s40850-021-0065-x
Franco M, Contreras C, Cortés P, Chappell MA, Soto-Gamboa M, Nespolo RF (2012) Aerobic power, huddling and the efficiency of torpor in the South American marsupial, Dromiciops gliroides. Biol Open 1:1178–1184. https://doi.org/10.1242/bio.20122790
Gager Y (2018) Information transfer about food as a reason for sociality in bats. Mamm Rev 49:113–120. https://doi.org/10.1111/mam.12146
Geiser F (2021) Ecological physiology of daily torpor and hibernation. Springer, Switzerland
Geiser F, Broome LS (1993) The effect of temperature on the pattern of torpor in a marsupial hibernator. J Comp Physiol B 163:133–137. https://doi.org/10.1007/BF00263598
Geiser F, Brigham RM (2000) Torpor, thermal biology, and energetics in Australian long-eared bats (Nyctophilus). J Comp Physiol B 170:153–162. https://doi.org/10.1007/s003600050270
Geiser F, Turbill C (2009) Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96(10):1235–1240. https://doi.org/10.1007/s00114-009-0583-0
Geiser F, Stawski C, Doty AC, Cooper CE, Nowack J (2018) A burning question: what are the risks and benefits of mammalian torpor during and after fires? Conserv Physiol 6:coy057. https://doi.org/10.1093/conphys/coy057
Haarsma A-J, Kaal R (2016) Predation of wood mice (Apodemus sylvaticus) on hibernating bats. Popul Ecol 58:567–576. https://doi.org/10.1007/s10144-016-0557-y
Heldmaier G, Ortmann S, Elvert R (2004) Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol 141:317–329. https://doi.org/10.1016/j.resp.2004.03.014
Humphries MM, Thomas DW, Kramer DL (2003) The role of energy availability in mammalian hibernation: a cost-benefit approach. Physiol Biochem 76:165–179. https://doi.org/10.1086/367949
Jonasson KA, Willis CKR (2012) Hibernation energetics of free-ranging little brown bats. J Exp Biol 215:2141–2149. https://doi.org/10.1242/jeb.066514
Kurta A, Bell GP, Nagy KA, Kunz TH (1989) Energetics of pregnancy and lactation in free-ranging little brown bats (Myotis lucifugus). Physiol Zool 62:804–818
Landes J, Pavard S, Henry P-Y, Terrien J (2020) Flexibility is costly: hidden physiological damage from seasonal phenotypic transitions in heterotheric species. Front Physiol 11:985. https://doi.org/10.3389/fphys.2020.00985
Lighton JRB (2018) Measuring metabolic rates. A manual for scientists. Oxford University Press, New York. https://doi.org/10.1093/oso/9780198830399.001.0001
Liow LH, Fortelius M, Lintulaakso K, Mannila H, Stenseth NC (2009) Lower extinction risk in sleep-or-hide mammals. Am Nat 173:264–272. https://doi.org/10.1086/595756
Matheson AL, Campbell KL, Willis CKR (2010) Feasting, fasting and freezing: energetic effects of meal size and temperature on torpor expression by little brown bats Myotis lucifugus. J Exp Biol 213:2165–2173. https://doi.org/10.1242/jeb.040188
Mayberry HW, McGuire LP, Willis CKR (2017) Body temperatures of hibernating little brown bats reveal pronounced behavioural activity during deep torpor and suggest a fever response during white-nose syndrome. J Comp Physiol B. https://doi.org/10.1007/s00360-017-1119-0
McGuire LP, Fuller NW, Dzal YA, Haase CG, Silas KA, Willis CKR, Olson SH, Lausen CL (2021) Similar hibernation physiology in bats across broad geographic ranges. J Comp Physiol B. https://doi.org/10.1007/s00360-021-01400-x
McLean JA, Speakman JR (2000) Effects of body mass and reproduction on the basal metabolic rate of Brown long-eared bats (Plecotus auritus). Physiol Biochem 73:112–121. https://doi.org/10.1086/316715
Michaelsen TC, Jensen KH, Högstedt G (2011) Topography is a limiting distributional factor in the soprano pipistrelle at its latitudinal extreme. Mamm Biol 76:295–301. https://doi.org/10.1016/j.mambio.2010.12.004
Michaelsen TC, Jensen KH, Högstedt G (2018) Does light condition affect the habitat use of soprano pipistrelles Pipistrellus pygmaeus at the species northern extreme? Acta Chiropt 20:377–385. https://doi.org/10.3161/15081109
Millesi E, Prossinger H, Dittami JP, Fieder M (2001) Hibernation effects on memory in European ground squirrels (Spermophilus citellus). J Biol Rhythms 16:264–271. https://doi.org/10.1177/074873040101600309
Muff S, Nilsen EB, O’Hara RB, Nater CR (2022) Rewriting results sections in the language of evidence. Trends Ecol Evol 37:203–210. https://doi.org/10.1016/j.tree.2021.10.009
Muggeo VMR (2008) Segmented: an R package to fit regression models with broken-line relathionships. R News 8:20–25
Pretzlaf I, Radchuk V, Turner JM, Dausmann KH (2021) Flexibility in thermal physiology and behaviour allows body mass maintenance in hibernating hazel dormice. J Zool. https://doi.org/10.1111/jzo.12862
Reher S, Dausmann KH (2021) Tropical bats counter heat by combining torpor with adaptive hyperthermia. Proc R Soc 288:20202059. https://doi.org/10.1098/rspb.2020.2059
Reher S, Rabarison H, Montero BK, Turner JM, Dausmann KH (2022) Disparate roost sites drive intraspecific physiological variation in a Malagasy bat. Oecologia 198(35):52. https://doi.org/10.1007/s00442-021-05088-2
Ruf T, Geiser F (2015) Daily torpor and hibernation in birds and mammals. Biol Rev 90:891–926. https://doi.org/10.1111/brv.12137
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment. Cambridge University Press, Cambridge
Selås V, Kobro S, Sonerud GA (2013) Population fluctuations of moths and small rodents in relation to plant production indices in southern Norway. Ecosphere 4:123. https://doi.org/10.1890/ES13-00228.1
Speakman JR, Webb PI, Racey PA (1991) Effects of disturbance on the energy expenditure of hibernating bats. J Appl Ecol 28:1087–1104
Stawski C, Geiser F (2010) Fat and fed: frequent use of summer torpor in a subtropical bat. Naturwissenschaften 97:29–35. https://doi.org/10.1007/s00114-009-0606-x
Stawski C, Geiser F (2011) Do season and distribution affect thermal energetics of a hibernating bat endemic to the tropics and subtropics? Am J Physiol Regul Integr Comp Physiol 301:R542–R547. https://doi.org/10.1152/ajpregu.00792.2010
Stawski C, Willis CKR, Geiser F (2014) The importance of temporal heterothermy in bats. J Zool 292:86–100. https://doi.org/10.1111/jzo.12105
Thomas DW, Geiser F (1997) Periodic arousals in hibernating mammals: is evaporative water loss involved? Funct Ecol 11:585–591. https://doi.org/10.1152/ajpregu.00562.2001
Turbill C, Körtner G, Geiser F (2008) Timing of the daily temperature cycle affects the critical arousal temperature and energy expenditure of lesser long-eared bats. J Exp Biol 211:3871–3878. https://doi.org/10.1242/jeb.023101
Turbill C, Bieber C, Ruf T (2011) Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc R Soc B 278:3355–3363. https://doi.org/10.1098/rspb.2011.0190
Ulrich J (2021) TTR: Technical trading rules. R package version 0.24.3. https://CRAN.R-project.org/package=TTR. Accessed 18 May 2022
Webb PI, Hays GC, Speakman JR, Racey PA (1992) The functional significance of ventilation frequency, and its relationship to oxygen demand in the resting brown long-eared bat, Plecotus auritus. J Comp Physiol B 162:144–147. https://doi.org/10.1007/bf00398339
Wermundsen T, Siivonen Y (2010) Seasonal variation in use of winter roosts by five bat species in south-east Finland. Cent Eur J Biol 5:262–273. https://doi.org/10.2478/s11535-009-0063-8
Willis CKR, Turbill C, Geiser F (2005) Torpor and thermal energetics in a tiny Australian vespertilionid, the little forest bat (Vespadelus vulturnus). J Comp Physiol B 175:479–486. https://doi.org/10.1007/s00360-005-0008-0
Willis CKR, Jameson JW, Faure PA, Boyles JG, Brack V Jr, Cervone TH (2009) Thermocron iButton and iBBat temperature dataloggers emit ultrasound. J Comp Physiol B 179:867–874. https://doi.org/10.1007/s00360-009-0366-0
Wilson DE, Mittermeier RA (eds) (2019) Handbook of the Mammals of the World, vol 9, Bats. Lynx Editions, Barcelona, pp 1008. ISBN: 978-84-16728-19-0
Wojciechowski MS, Jefimow M, Tegowska E (2007) Environmental conditions, rather than season, determine torpor use and temperature selection in large mouse-eared bats (Myotis myotis). Comp Biochem Physiol A 147:828–840. https://doi.org/10.1016/j.cbpa.2006.06.039
Wolf BO, McKechnie AE, Schmitt CJ, Czenze ZJ, Johnson AB, Witt CC (2020) Extreme and variable torpor among high-elevation Andean hummingbird species. Biol Lett 16:20200428. https://doi.org/10.1098/rsbl.2020.0428
Zervanos SM, Maher CR, Waldvogel JA, Florant GL (2010) Latitudinal differences in the hibernation characteristics of Woodchucks (Marmota monax). Physiol Biochem 83:135–141. https://doi.org/10.1086/648736
Zervanos SM, Maher CR, Florant GL (2013) Effects of body mass on hibernation strategies of woodchucks (Marmota monax). ICB 54:443–451. https://doi.org/10.1093/icb/ict100
Acknowledgements
We thank three anonymous reviewers for constructive feedback on the first draft of this manuscript. Additionally, we thank Helene M. Hannestad and Håvard A. Hald for assistance during the fieldwork. The Norwegian Environment Agency and the Norwegian Food Safety Authority granted permission to capture and hold bats. We also thank the Norwegian University of Science and Technology for financial support for the study.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital). Funding for this research was provided by the Norwegian University of Science and Technology.
Author information
Authors and Affiliations
Contributions
RS and CS framed the idea of the experiment. RS, MAF, and JK collected the data. RS and KHS performed the preliminary analysis of the data. RS wrote the initial draft. CS acquired the funding for the project. CS, KE, CB and JK provided additional resources. All authors participated in the writing and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the authorship of this article. Use of product or corporation names is for descriptive purposes only and implies no endorsement by any author or affiliation.
Ethics approval
Permits for the capture and handling of P. auritus were granted by the Norwegian Food Safety Authority (FOTS ID 23284) and the Norwegian Environment Agency (ref. 2018/4899).
Additional information
Communicated by G. Heldmaier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sørås, R., Fjelldal, M.A., Bech, C. et al. State dependence of arousal from torpor in brown long-eared bats (Plecotus auritus). J Comp Physiol B 192, 815–827 (2022). https://doi.org/10.1007/s00360-022-01451-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-022-01451-8