Abstract
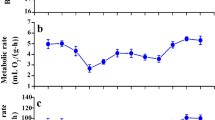
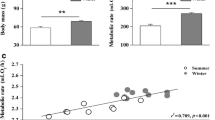
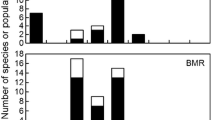
In birds, acclimation and acclimatization to temperature are associated with changes in basal (BMR), summit (Msum) and maximal (MMR) metabolic rates but little is known about the rate at which species adjust their phenotype to short-term temperature variations. Our aims were (1) to determine the pattern of metabolic adjustments following a rapid temperature change, (2) to determine whether performance varies at similar rates during exposure to warm or cold environments, and (3) to determine if BMR, Msum and MMR change at comparable rates during thermal acclimation. We measured these parameters in white-throated sparrows (Zonotrichia albicollis), black-capped chickadees (Poecile atricapillus), and snow buntings (Plectrophenax nivalis) after acclimation to 10 °C (day 0) and on the 4th and 8th days of acclimation to either −5 or 28 °C. Birds changed their metabolic phenotype within 8 days with patterns differing among species. Sparrows expressed the expected metabolic increases in the cold and decreases at thermoneutrality while performance in chickadees and buntings was not influenced by temperature but changed over time with inverse patterns. Our results suggest that BMR varies at comparable rates in warm and cold environments but changes faster than Msum and MMR, likely due to limitations in the rate of change in organ size and function. They also suggest that maximal metabolic capacity is lost faster in a warm environment than it is gained in a cold environment. With the expected increase in temperature stochasticity at northern latitudes, a loss of thermogenic capacity during warm winter days could, therefore, be detrimental if birds are slow to readjust their phenotype with the return of cold days.






Similar content being viewed by others
References
Barcelo G, Salinas J, Cavieres G, Canals M, Sabat P (2009) Thermal history can affect the short-term thermal acclimation of basal metabolic rate in the passerine Zonotrichia capensis. J Therm Biol 34:415–419
Bartholomew GA, Vleck D, Vleck CM (1981) Instantaneous measurements of oxygen consumption during pre-flight warm-up and post-flight cooling in sphingid and saturniid moths. J Exp Biol 90:17–32
Bauchinger U, McWilliams SR (2009) Carbon turnover in tissues of a passerine bird: allometry isotopic clocks and phenotypic flexibility in organ size. Physiol Biochem Zool 82:787–797
Bauchinger U, McWilliams SR (2010) Extent of phenotypic flexibility during long-distance flight is determined by tissue-specific turnover rates: a new hypothesis. J Avian Biol 41:603–608
Blem CR (1976) Patterns of lipid storage and utilization in birds. Am Zool 16:671–684
Broggi J, Hohtola E, Koivula K, Orell M, Thomson RL, Nilsson J-A (2007) Sources of variation in winter basal metabolic rate in the great tit. Funct Ecol 21:528–533
Bush NG, Brown M, Downs CT (2008) Effects of short-term acclimation on thermoregulatory responses of the rock kestrel Falco rupicolus. J Therm Biol 33:425–430
Busse P (2000) Bird station manual. Gdansk University Press, Gdansk
Cavieres G, Sabat P (2008) Geographic variation in the response to thermal acclimation in rufous-collared sparrows: are physiological flexibility and environmental heterogeneity correlated? Funct Ecol 22:509–515
Chappell MA, Bech C, Buttemer WA (1999) The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J Exp Biol 202:2269–2279
Cooper SJ (2002) Seasonal metabolic acclimatization in mountain chickadees and juniper titmice. Physiol Biochem Zool 75:386–395
Cooper SJ, Swanson DL (1994) Seasonal acclimatization of thermoregulation in the black-capped chickadee. Condor 96:638–646
Devost I, Hallot F, Milbergue M, Petit M, Vézina F (2014) Lipid metabolites as markers of fattening rate in a non-migratory passerine: effects of ambient temperature and individual variation. Comp Biochem Physiol A-Mol Integr Physiol 177:18–26
Dietz MW, Dekinga A, Piersma T, Verhulst S (1999) Estimating organ size in small migrating shorebirds with ultrasonography: an intercalibration exercise. Physiol Biochem Zool 72:28–37
Dietz MW, Piersma T, Hedenström A, Brugge M (2007) Intraspecific variation in avian pectoral muscle mass: constraints on maintaining manoeuvrability with increasing body mass. Funct Ecol 21:317–326
Dutenhoffer MS, Swanson DL (1996) Relationship of basal to summit metabolic rate in passerine birds and the aerobic capacity model for the evolution of endothermy. Physiol Zool 69:1232–1254
Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2000) Climate extremes: observations modeling and impacts. Science 289:2068–2074
Falls JB, Kopachena JG (2010) White-throated sparrow (Zonotrichia albicollis). The Birds of North America Online. Cornell Lab of Ornithology. http://bna.birds.cornell.edu/bna/species/128. Accessed 10 Dec 2015
Foote JR, Mennill DJ, Ratcliffe LM, Smith SM (2010) Black-capped chickadee (Poecile atricapillus) The Birds of North America Online. Cornell Lab of Ornithology. http://bna.birds.cornell.edu/bna/species/039. Accessed 10 Dec 2015
Gessaman JA, Nagy KA (1988) Energy metabolism: errors in gas-exchange conversion factors. Physiol Zool 61:507–513
Gosler AG (1996) Environmental and social determinants of winter fat storage in the great tit Parus major. J Anim Ecol 65:1–17
Hammond KA, Chappell MA, Cardullo RA, Lin R, Johnsen TS (2000) The mechanistic basis of aerobic performance variation in red junglefowl. J Exp Biol 203:2053–2064
IPCC (2013) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Katz RW, Brush GS, Parlange MB (2005) Statistics of extremes: modeling ecological disturbances. Ecology 86:1124–1134
Kersten M, Piersma T (1986) High levels of energy expenditure in shorebirds: metabolic adaptations to an energetically expensive way of life. Ardea 75:175–187
Klaassen M, Oltrogge M, Trost L (2004) Basal metabolic rate food intake and body mass in cold- and warm-acclimated garden warblers. Comp Biochem Physiol A-Mol Integr Physiol 137:639–647
Kontogiannis JE (1968) Effect of temperature and exercise on energy intake and body weight of the white-throated sparrow Zonotrichia albicollis. Physiol Zool 41:54–64
Krams I (2002) Mass-dependent take-off ability in wintering great tits (Parus major): comparison of top-ranked adult males and subordinate juvenile females. Behav Ecol Sociobiol 51:345–349
Kullberg C, Fransson T, Jakobsson S (1996) Impaired predator evasion in fat blackcaps (Sylvia atricapilla). Proc R Soc B 263:1671–1675
Lighton JRB (2008) Measuring metabolic rates: a manual for scientists. Oxford University Press, New York
Liknes ET, Swanson DL (1996) Seasonal variation in cold tolerance basal metabolic rate and maximal capacity for thermogenesis in white-breasted nuthatches Sitta carolinensis and downy woodpeckers Picoides pubescens two unrelated arboreal temperate residents. J Avian Biol 27:279–288
Liknes ET, Swanson DL (2011a) Phenotypic flexibility of body composition associated with seasonal acclimatization in passerine birds. J Therm Biol 36:363–370
Liknes ET, Swanson DL (2011b) Phenotypic flexibility in passerine birds: seasonal variation of aerobic enzyme activities in skeletal muscle. J Therm Biol 36:430–436
Liknes ET, Scott SM, Swanson DL (2002) Seasonal acclimatization in the american goldfinch revisited: to what extent do metabolic rates vary seasonally? Condor 104:548–557
Lind J, Fransson T, Jakobsson S, Kullberg C (1999) Reduced take-off ability in robins (Erithacus rubecula) due to migratory fuel load. Behav Ecol Sociobiol 46:65–70
Lindström Å, Visser GH, Daan S (1993) The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol Zool 66:490–510
Liu JS, Li M (2006) Phenotypic flexibility of metabolic rate and organ masses among tree sparrows Passer montanus in seasonal acclimatization. Acta Zool Sin 52:469–477
Maldonado KE, Cavieres G, Veloso C, Canals M, Sabat P (2008) Physiological responses in rufous-collared sparrows to thermal acclimation and seasonal acclimatization. J Comp Physiol B 179:335–343
Mandin C, Vézina F (2012) Daily variation in markers of nutritional condition in wintering black-capped chickadees Poecile atricapillus. Ibis 154:791–802
Marsh RL, Dawson WR (1989) Avian adjustments to cold. In: Wang LCH (ed) Advances in comparative and environmental physiology, vol 4., animal adaptation to coldSpringer, Berlin, pp 205–253
McKechnie AE (2008) Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J Comp Physiol B 178:235–247
McKechnie AE, Swanson DL (2010) Sources and significance of variation in basal summit and maximal metabolic rates in birds. Curr Zool 56:741–758
McKechnie AE, Chetty K, Lovegrove BG (2007) Phenotypic flexibility in the basal metabolic rate of laughing doves: responses to short-term thermal acclimation. J Exp Biol 210:97–106
McWilliams SR, Karasov WH (2014) Spare capacity and phenotypic flexibility in the digestive system of a migratory bird: defining the limits of animal design. Proc R Soc B 281:1–9
Metcalfe J, Schmidt KL, Kerr WB, Guglielmo CG, MacDougall-Shackleton SA (2013) White-throated sparrows adjust behaviour in response to manipulations of barometric pressure and temperature. Anim Behav 86:1285–1290
Montgomerie R, Lyon B (2011) Snow bunting (Plectrophenax nivalis) The Birds of North America Online. Cornell Lab of Ornithology. http://bna.birds.cornell.edu/bna/species/198. Accessed 10 Dec 2015
Naugler CT (2014) American tree sparrow (Spizella arborea). The Birds of North America Online. Cornell Lab of Ornithology. http://bna.birds.cornell.edu/bna/species/037. Accessed 10 Dec 2015
Nolan V, Jr Ketterson ED, Cristol DA, Rogers CM, Clotfelter ED, Titus RC, Schoech SJ, Snajdr E (2002) Dark-eyed junco (Junco hyemalis). The Birds of North America Online. Cornell Lab of Ornithology. http://bna.birds.cornell.edu/bna/species/716. Accessed 10 Dec 2015
O’Connor TP (1995) Metabolic characteristics and body composition in house finches: effects of seasonal acclimatization. J Comp Physiol B 165:298–305
Odum EP (1949) Weight variations in wintering white-throated sparrows in relation to temperature and migration. Wilson Bull 61:3–14
Pena-Villalobos I, Nunez-Villegas M, Bozinovic F, Sabat P (2014) Metabolic enzymes in seasonally acclimatized and cold acclimated rufous-collared sparrow inhabiting a Chilean Mediterranean environment. Curr Zool 60:338–350
Petit M, Vézina F (2013) Phenotype manipulations confirm the role of pectoral muscles and haematocrit in avian maximal thermogenic capacity. J Exp Biol 217:824–830
Petit M, Vézina F (2014) Reaction norms in natural conditions: how does metabolic performance respond to weather variations in a small endotherm facing cold environments? Plos One. doi: 101371/journalpone 0113617
Petit M, Vézina F, Piersma T (2010) Ambient temperature does not affect fuelling rate in absence of digestive constraints in long-distance migrant shorebird fuelling up in captivity. J Comp Physiol B 180:847–856
Petit M, Lewden A, Vézina F (2013) Intra-seasonal flexibility in avian metabolic performance highlights the uncoupling of basal metabolic rate and thermogenic capacity. Plos One. doi: 101371/journalpone0068292
Petit M, Lewden A, Vézina F (2014) How does flexibility in body composition relate to seasonal changes in metabolic performance in a small passerine wintering at northern latitude? Physiol Biochem Zool 87:539–549
Piersma T (2011) Why marathon migrants get away with high metabolic ceilings: towards an ecology of physiological restraint. J Exp Biol 214:295–302
Piersma T, Bruinzeel L, Drent R, Kersten M, VanderMeer J, Wiersma P (1996) Variability in basal metabolic rate of a long-distance migrant shorebird (red knot Calidris canutus) reflects shifts in organ sizes. Physiol Zool 69:191–217
Price ER, Guglielmo CG (2009) The effect of muscle phospholipid fatty acid composition on exercise performance: a direct test in the migratory white-throated sparrow (Zonotrichia albicollis). Am J Physiol 297:R775–R782
Rezende EL, Chappell MA, Hammond KA (2004) Cold-acclimation in Peromyscus: temporal effects and individual variation in maximum metabolism and ventilatory traits. J Exp Biol 207:295–305
Royer-Boutin P, Cortés P, Milbergue M, Petit M, Vézina F (2015) Estimation of muscle mass by ultrasonography differs between observers and life states of models in small birds. Physiol Biochem Zool 88:336–344
Saarela S, Hohtola E (2003) Seasonal thermal acclimatization in sedentary and active pigeons. Isr J Zool 49:185–193
Sabat P, Cavieres G, Veloso C, Canals M, Bozinovic F (2009) Intraspecific basal metabolic rate varies with trophic level in rufous-collared sparrows. Comp Biochem Physiol A-Mol Integr Physiol 154:502–507
Scholander PF, Hock R, Walters V, Johnson F, Irving L (1950) Heat regulation in some arctic and tropical mammals and birds. Biol Bull 99:237–258
Scott I, Evans PR (1992) The metabolic output of avian (Sturnus vulgaris, Calidris alpina) adipose tissue liver and skeletal muscle: implications for BMR/body mass relationships. Comp Biochem Physiol A Physiol 103:329–332
Seibert HC (1949) Differences between migrant and non-migrant birds in food and water intake at various temperatures and photoperiods. Auk 66:128–153
Swanson DL (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr Ornithol 17:75–129
Swanson DL, Dean KL (1999) Migration-induced variation in thermogenic capacity in migratory passerines. J Avian Biol 30:245–254
Swanson DL, Merkord C (2012) Seasonal phenotypic flexibility of flight muscle size in small birds: a comparison of ultrasonography and tissue mass measurements. J Ornithol 154:119–127
Swanson DL, Olmstead KL (1999) Evidence for a proximate influence of winter temperature on metabolism in passerine birds. Physiol Biochem Zool 72:566–575
Swanson DL, Vézina F (2015) Environmental ecological and mechanistic drivers of avian seasonal metabolic flexibility in response to cold winters. J Ornithol 156:377–388
Swanson DL, Drymalski MW, Brown JR (1996) Sliding vs static cold exposure and the measurement of summit metabolism in birds. J Therm Biol 21:221–226
Swanson DL, Thomas NE, Liknes ET, Cooper SJ (2012) Intraspecific correlations of basal and maximal metabolic rates in birds and the aerobic capacity model for the evolution of endothermy. Plos One. doi: 101371/journalpone0034271
Swanson DL, Zhang Y, King MO (2013) Individual variation in thermogenic capacity is correlated with flight muscle size but not cellular metabolic capacity in american goldfinches (Spinus tristis). Physiol Biochem Zool 86:421–431
Swanson DL, Zhang Y, Liu JS, Merkord CL, King MO (2014a) Relative roles of temperature and photoperiod as drivers of metabolic flexibility in dark-eyed juncos. J Exp Biol 217:866–875
Swanson DL, Zhang Y, King MO (2014b) Mechanistic drivers of flexibility in summit metabolic rates of small birds. Plos One. doi: 101371/journalpone0101577
Tieleman BI, Williams JB, Bloomer P (2003) Adaptation of metabolism and evaporative water loss along an aridity gradient. Proc R Soc B 270:207–214
Vaillancourt E, Prud’Homme S, Haman F, Guglielmo CG, Weber JM (2005) Energetics of a long-distance migrant shorebird (Philomachus pugnax) during cold exposure and running. J Exp Biol 208:317–325
van de Ven T, Mzilikazi N, McKechnie AE (2013) Phenotypic flexibility in body mass basal metabolic rate and summit metabolism in southern red bishops (Euplectes orix): responses to short term thermal acclimation. Comp Biochem Physiol A-Mol Integr Physiol 165:319–327
Vézina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European Starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Ex Biol 209:3141–3154
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2007) Thermogenic side effects to migratory predisposition in shorebirds. Am J Physiol 292:R1287–R1297
Vézina F, Gustowska A, Jalvingh KM, Chastel O, Piersma T (2009) Hormonal correlates and thermoregulatory consequences of molting on metabolic rate in a northerly wintering shorebird. Physiol Biochem Zool 82:129–142
Vézina F, Dekinga A, Piersma T (2011) Shorebirds’ seasonal adjustments in thermogenic capacity are reflected by changes in body mass: how preprogrammed and instantaneous acclimation work together. Integr Comp Biol 51:394–408
Wiersma P, Chappell MA, Williams JB (2007) Cold- and exercise-induced peak metabolic rates in tropical birds. Proc Natl Acad Sci USA 104:20866–20871
Williams JB, Tieleman BI (2000) Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperatures. J Exp Biol 203:3153–3159
Zhang Y, Eyster K, Liu JS, Swanson DL (2015a) Cross-training in birds: cold and exercise training produce similar changes in maximal metabolic output muscle masses and myostatin expression in house sparrows (Passer domesticus). J Exp Biol 218:2190–2200
Zhang Y, King MO, Harmon E, Swanson DL (2015b) Summer-to-winter phenotypic flexibility of fatty acid transport and catabolism in skeletal muscle and heart of small birds. Physiol Biochem Zool 88:535–549
Zheng WH, Li M, Liu JS, Shao SL (2008) Seasonal acclimatization of metabolism in eurasian tree sparrows (Passer montanus). Comp Biochem Physiol A-Mol Integr Physiol 151:519–525
Zhu W-L, Cai J-H, Lian X, Wang Z-K (2010) Adaptive character of metabolism in Eothenomys miletus in Hengduan Mountains region during cold acclimation. J Therm Biol 35:417–421
Acknowledgments
We would like to thank the Corporation de la Forêt d’enseignement et de recherche Macpès who granted us access to their field facilities for capturing birds. We are also grateful to Alexandre Anctil and Ludovic Jolicoeur for their assistance during captures of snow buntings. A special thanks goes to David Swanson and Yufeng Zhang from the University of South Dakota for training KD with MMR and to Richard Lafrance from UQAR for building our hop-flutter wheel. We are also grateful to David Swanson and Joël Bêty for commenting a previous version of this paper as well as to two anonymous reviewers for their constructive comments. We thank Catherine Burman-Plourde and Josianne Ruest for their help with food intake measurements and bird maintenance as well as Magali Petit and Myriam Milbergue for their advice and constant support. This research was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) as well as a Leader Opportunity Fund from the Canadian Foundation for Innovation to FV. FH received an Industrial Innovation Scholarship from the Fonds de Recherche du Québec Nature et les Technologies (FRQNT) and KD received an Alexander Graham Bell Graduate Scholarship from NSERC and well as a Master’s Scholarship from FRQNT during this study. KD was also supported by a Mobility Fellowship from EnviroNorth during her training in Dr. Swanson’s lab.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All bird manipulations were approved by the Université du Québec à Rimouski animal care committee (CPA-52-13-113) and have been conducted under scientific (SC-47) and banding (10704D) permits from Environment Canada - Canadian Wildlife Service.
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Dubois, K., Hallot, F. & Vézina, F. Basal and maximal metabolic rates differ in their response to rapid temperature change among avian species. J Comp Physiol B 186, 919–935 (2016). https://doi.org/10.1007/s00360-016-1001-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1001-5