Abstract
Background and Purpose
The present study aims to investigate the effects of external neuromuscular electrical stimulation (NMES) on urinary symptoms, pelvic floor muscle strength (PFMS), quality of life (QoL), sexual function, perception of subjective improvement (PSI), and satisfaction in urgency urinary incontinence (UUI).
Materials and Methods
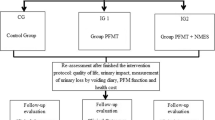
The randomized sham-controlled study design was employed in this study. Women aged 18–65 years, who were diagnosed with UUI, were randomly allocated into the NMES (external NMES + lifestyle advice, n = 15) and sham groups (sham NMES + lifestyle advice, n = 15). Both groups performed the application for 30 min, three days a week for eight weeks. Urinary symptoms were evaluated by using the International Incontinence Consultation Questionnaire-Short Form (ICIQ-SF) and a 3-day bladder diary. PFMS was assessed using the Modified Oxford Scale (MOS), QoL using the King’s Health Questionnaire (KHQ), and sexual function using the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ-12). The PSI and satisfaction were questioned.
Results
There was a higher level of decrease in the ICIQ-SF score, the mean number of voids/night and UI, all scores related to the KHQ (excluding interpersonal relationships), and a higher level of increase in maximum voiding volume, MOS scores, PISQ-12-emotional, PISQ-12-physical, and PISQ-12-total scores in the NMES group when compared to the sham group (p < 0.05). PSI and satisfaction were at higher levels in the NMES group than in the sham group (p < 0.05).
Conclusions
External NMES was an effective and complementary method in reducing urinary symptoms and improving PFMS, QoL, sexual function, PSI, and satisfaction level in women with UUI.
Clinical Trial Registration
NCT04727983.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urgency urinary incontinence (UUI), which is the involuntary loss of urine associated with urgency, constitutes 22% of urinary incontinence (UI) in women [1]. The etiopathogenesis of UUI generally includes detrusor overactivity, low detrusor compliance, urothelial sensitivity, and bladder hypersensitivity [1]. It negatively affects the quality of life (QoL) [2].
Conservative treatments, including lifestyle advice, bladder training, pelvic floor muscle exercise (PFME), and electrical stimulation (ES), can be used [1]. Lifestyle advice, such as reducing bladder irritants, weight control, and increasing physical activity, is considered a method for treating UUI according to guidelines [3, 4].
Neuromuscular electrical stimulation (NMES) can improve sensorial awareness, muscle reeducation, and circulation and can be applied transcutaneously, percutaneously, or as an implant [5]. Transcutaneous applications are divided into internal (IES) and external ES (EES). However, external NMES devices, known as novel EES, are preferred more than IES due to disadvantages such as expensive probes, risk of infection, and patient reluctance towards intravaginal-anal application. The number of studies examining the effects of NMES on overactive bladder (OAB) accompanied by UUI is limited [6]. More high-quality studies examining the effects of external NMES on patients with UUI are needed.
Therefore, the present study aims to examine the effects of external NMES on urinary symptoms, pelvic floor muscle strength (PFMS), QoL, sexual function, perception of subjective improvement (PSI), and satisfaction in women with UUI.
Methods
Study design
This study, which was planned as a randomized sham-controlled trial, was approved by the clinical research ethics committee of Recep Tayyip Erdogan University (Approval number:2020/07). The Declaration of Helsinki was followed in the research process. The present study, which was carried out at the gynecology and obstetrics outpatient clinic of training and research hospital of the university, was registered at https://www.clinicaltrials.gov (NCT04727983).
Participants
Volunteer women aged 18–65 years, who were newly diagnosed with UUI or discontinued their drug use for UUI at least one month ago, were included in the present study. Exclusion criteria were pregnancy, urinary infection, advanced pelvic organ prolapse (stage 3 and above [7]), stress urinary incontinence (SUI) (positive cough stress test), mixed urinary incontinence, neurological disease (i.e. Parkinson’s Disease, multiple sclerosis, etc.), loss of sensation involving pelvic region and lower extremity, cardiac arrhythmia, using electronic/metal implants or pacemaker and malignant disease. Written consent forms were obtained.
Results
Physical and demographic characteristics (age and body mass index (BMI)) and clinic histories (number of pregnancies, number of deliveries, constipation) were recorded. Urinary infection was screened using urine analysis at the time of enrollment. No infection was detected in any patient during treatment. Sensorial evaluations were conducted around the thigh, where ES application would be performed. There was no patient describing neurological symptoms or sensational loss. All assessments were conducted by the same therapist before treatment (BT), mid-term (MT, 4th week), and after treatment (AT, 8th week). AT, PSI, and satisfaction level of patients were also questioned.
The urinary incontinence severity was evaluated as the primary outcome by using the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-SF) [8]. Moreover, the participants completed a 3-day bladder diary. The mean number of voids/day, voids/night, UI, and maximum voiding volume were recorded for the urinary symptoms. The PFMS was assessed using the Modified Oxford Scale (MOS) while the woman was in the lithotomy position [9]. The QoL was examined by using the first part of the King’s Health Questionnaire (KHQ), including 9 sub-dimensions [10]. The sexual function was addressed by using the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Scale (PISQ-12) [11]. The PSI of patients was evaluated through a 4-item Likert-type scale (worse, same, better, cured) [12]. Patient satisfaction was questioned by using a Visual Analog Scale (VAS) consisting of a horizontal line of 10 cm in length, where “0” defines “I am not at all satisfied with the treatment” and “10” defines “very satisfied with the treatment”.
Randomization
Participants were assigned to one of NMES and sham groups by using a computer-based block randomization list, which was created by an individual not involved in the assessment and the treatment.
Intervention
External NMES and lifestyle advice were given to the NMES group, whereas sham NMES and lifestyle advice were given to the sham group. Patients received advice on fluid intake, diet, bladder irritants, constipation, and weight control by using a brochure. Compliance with these lifestyle advices was assessed using a 4-item Likert-type scale. None of the patients used medication during the study.
An INNOVO® device (Atlantic Therapeutics, Galway, Ireland) consisting of 2 wearable sleeves and 8 external electrodes was used in NMES application (Fig. 1). The procedure was conducted in a back-supported sitting position with a specific waveform and parameters (10 Hz frequency, 250 µs pulse width, 0.5-s ramp up and down ramp time, 5-s contraction, and 0-s relaxation periods). In the Sham NMES, the electrodes were connected as in the NMES group and the light and the sound of the device were turned on, but the current was not increased. Patients were blinded to the intervention modalities administered to the groups. Both groups received the interventions for 30 min, three days a week for eight weeks.
Sample size and statistical analysis
G*Power (v.3.9.1.7) program was used to determine the sample size. A pilot study carried out on 10 women revealed effect sizes for ICIQ-SF, MOS, KHQ-incontinence effect, and PISQ-total scores as 0.675, 0.670, 0.519, and 0.557, respectively. At least 14 patients per group were needed at 90% power (α = 0.05, β = 0.10). An additional 20% of patients were added to account for data losses, resulting in a total of at least 34 patients for the study.
The normal distribution of variables was determined using the Shapiro–Wilk test. Mixed ANOVA was used to analyze the effects of the group factor (NMES and sham group), time factor (BT, MT, and AT), and their interaction. Pairwise comparisons were conducted for the time factor by using the Bonferroni method. The Chi-Square test was used for categorical variables. Statistical analyses were conducted by using IBM SPSS Statistics 21.0, and the statistical significance was set at p < 0.05.
Results
Forty-four patients were evaluated for eligibility, and 10 patients were excluded for various reasons (advanced pelvic organ prolapse (n = 5), urinary infection (n = 4), urinary retention (n = 1)). After randomization, 1 patient from the NMES group and 2 patients from the sham group withdrew due to COVID-19, and 1 patient from the NMES group withdrew due to relocation. Fifteen patients in the NMES group (age = 47.66 ± 9.59 years, BMI = 28.32 ± 5.93 kg/m2, number of pregnancies = 4, number of deliveries = 3, constipation = 8) and 15 patients in the sham group (age = 48.06 ± 9.85 years, BMI = 32.82 ± 6.67 kg/m2, number of pregnancies = 3, number of deliveries = 3, constipation = 7) completed the study. Physical, demographic, and clinical characteristics, urinary symptoms, PFMS, QoL, sexual function, PSI, and satisfaction results were similar between groups at baseline (p > 0.05). No adverse effects were reported during the applications.
Urinary symptoms
A reduction was found in the ICIQ-SF scores between BT-MT, BT-AT, and MT-AT in the NMES group (p < 0.05), while there was a decrease in the ICIQ-SF between BT-AT in the sham group (p < 0.05). There was a higher level of decrease in the ICIQ-SF scores in the NMES group when compared to the sham group (for group*time interaction (GTI) p < 0.05, Table 1).
In the NMES group, there was a decrease in the number of voids per day and night (BT-MT and BT-AT) and the number of urinary incontinences (UI) (BT-MT and BT-AT). Additionally, an increase was observed in maximum voiding volume between BT-AT and MT-AT (p < 0.05). In the sham group, a decrease was found between BT-MT and BT-AT in only the mean number of UI (p < 0.05). A higher level of decrease was observed in the number of voids/night and the number of UI and a higher level of increase in maximum voiding volume in the NMES group when compared to the sham group (for GTI p < 0.05, Table 1).
PFMS
An increase was found in MOS scores between BT-MT, BT-AT, and MT-AT in the NMES group (p < 0.05). No improvement was found in the sham group (p > 0.05). A higher level of increase was observed in MOS scores in the NMES group when compared to the sham group (for GTI p < 0.05, Table 1).
QoL
A decrease was determined in KHQ-general health, KHQ-social and KHQ-personal relations limitation scores of KHQ between BT-AT and MT-AT and also in KHQ-incontinence effect, KHQ-physical and KHQ-role limitation scores, KHQ-emotional status, KHQ-sleep-energy disturbance, and KHQ-severity scores between BT-MT, BT-AT, and MT-AT in the NMES group (p < 0.05). No change was observed in the sham group (p > 0.05). There was a higher level of decrease in KHQ scores (excluding interpersonal relationships) in the NMES group than in the sham group (for GTI p < 0.05, Table 2).
Sexual function
An increase was determined in PISQ-12-emotional and PISQ-12-total scores between BT-MT, BT-AT, and MT-AT and in PISQ-12-physical scores between BT-AT and MT-AT in the NMES group (p < 0.05). No improvement was observed in the sham group (p > 0.05). There was a higher level of increase in PISQ-12 scores (excluding partner dependent score) in the NMES group than in the sham group (for GTI p < 0.05, Table 1).
PSI and satisfaction
PSI results showed that in the NMES group, 60.0% gave the response “I am better” and 40.0% gave the response “I am completely healed”. In the sham group, 53.3% responded by stating “I am the same”, 40.0% by stating “better”, and 6.7% by stating “fully recovered”. There was a significant difference between groups in PSI (p < 0.001), and patient satisfaction was higher in the NMES group (p < 0.001). Regarding compliance with recommendations, 6.7% in the NMES group sometimes applied them, 86.7% applied them mostly, and 6.7% applied them completely. In the sham group, 26.7% sometimes applied them, 66.7% applied them mostly, and 6.7% applied them completely. There was no significant difference between groups in compliance with recommendations (p > 0.05).
Discussion
The present study revealed that the NMES was effective in reducing urinary symptoms and improving PFMS, QoL, sexual function, PSI, and patient satisfaction levels. There was a higher level of improvement in some urinary symptoms, PFMS, QoL, and sexual function in the NMES group in the early period (MT-4th week) than in the sham group. In the sham group, only urinary symptoms were reduced in the MT and AT.
Generally, low-frequency currents are used in ES applications in the management of UUI [13,14,15]. Yamanishi et al. [13] divided OAB patients with UUI into ES and sham ES groups. After treatment, UI, urgency, and voiding episodes decreased, and bladder capacity improved in the NMES group more than in the sham group. Franzen et al. [14] compared drug therapy with IES for UUI, finding similar improvements in voiding frequency and bladder capacity. Guo et al. [15] divided stroke patients into two groups and reported that NMES significantly reduced symptoms of UUI in comparison to the sham group. It was found in the present study that NMES decreased incontinence severity and frequency, as well as day and night urination, while increasing maximum voided volume. These results may be because the low-frequency NMES applications stimulating the pudendal nerve afferents could activate the detrusor muscle reflexively, while also reducing detrusor hypersensitivity through sensory awareness and cortical or neural changes [16].
Decreased PFMS may be a risk factor for UUI symptoms [17]. McClurg et al. [18] reported that NMES (40 Hz) with intravaginal probe plus PFME was more effective than sham NMES in reducing incontinence symptoms in UUI patients but had similar effects on PFMS. Celenay et al. [6] stated that external NMES application improved PFMS in women with OAB-wet. In a systematic review, it was reported that NMES could increase PFMS more than PFME [19]. In the present study, PFMS improved in only the NMES group in MT and AT. These results may have originated from low-frequency NMES, increasing blood flow, regeneration, and increasing sensory input [16]. Furthermore, patients with UUI tend to over-contract their pelvic floor muscles to suppress urgency, and thus pelvic floor functions may be impaired [20]. Accordingly, the reduction of UUI symptoms by external NMES may also have contributed to PFMS.
It is known that UUI has more negative effects on QoL than SUI [2]. Studies suggest both IES (5–10 Hz) and drug therapy improve QoL, but drug therapy may have side effects [14, 21]. In the present study, in the NMES group, it was observed that QoL increased in all sub-dimensions in AT, while some sub-dimensions also improved in MT. NMES applied at low frequency can help control urgency reflexively by stimulating pudendal nerve afferents [5, 16]. Thus, UUI can be decreased and QoL may be improved.
Women with UUI often report lubrication and dyspareunia as major complaints with a rate of 34% [22]. Dmochowski et al. [23] reported that both EES and IES had similar effects in reducing SUI and improving sexual function. In the present study, there was an improvement in all parameters related to sexual functions improved more in the NMES group when compared to the sham group, except for partner dependency. Sexual function can be affected by many factors such as physical and psychological factors and the relationship with the partner [21]. These improvements may be due to reducing UUI and improving the sensory awareness of the pelvic floor, blood flow, and vaginal secretion with NMES [24].
A higher level of improvement was found in the NMES group in terms of PSI and patient satisfaction. Furthermore, the PSI values were also high in the sham group. The improvement in sham group is thought to be due to the compliance with lifestyle recommendations, the patient-therapist interaction, and increased patients’ belief in treatment with a device [25].
In conclusion, the external NMES was effective and complementary when reducing urinary symptoms and improving PFMS, QoL, sexual function, PSI, and patient satisfaction in women with UUI. Since the device is affordable and the procedure can be easily conducted at home, it can be recommended to patients as a home program. Long-term follow-up studies examining the effects of external NMES in both men and women with different pelvic floor dysfunctions are needed.
Data Availability
All data is available in our system. Authors will be contacted for usage.
References
Nandy S, Ranganathan S (2022) Urge Incontinence. In:StatPearls. Treasure Island (FL): StatPearls Publishing 33085319
Chiaffarino F, Parazzini F, Lavezzari M et al (2003) Impact of urinary incontinence and overactive bladder on quality of life. Eur Urol 43(5):535–538. https://doi.org/10.1016/S0302-2838(03)00097-6
Imamura M, Williams K, Wells M, McGrother C (2015) Lifestyle interventions for the treatment of urinary incontinence in adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003505.pub5
Frawley H, Shelly B, Morin M et al (2021) An International Continence Society (ICS) report on the terminology for pelvic floor muscle assessment. Neurourol Urodyn 40(5):1217–1260. https://doi.org/10.1002/nau.24658
Weil EH, Matzel K (2015) Electrical stimulation for pelvic floor disorders. Martellucci J, (ed). Springer International Publishing https://doi.org/10.1007/978-3-319-06947-0_1
Celenay ST, Karaaslan Y, Coban O, Oskay K (2022) A comparison of Kinesio taping and external electrical stimulation in addition to pelvic floor muscle exercise and sole pelvic floor muscle exercise in women with overactive bladder: a randomized controlled study. Disabil Rehabil 44(18):5124–5132. https://doi.org/10.1080/09638288.2021.1925751
Zeiger BB, da Silva Carramão S, Del Roy CA et al (2022) Vaginal pessary in advanced pelvic organ prolapse: impact on quality of life. Int Urogynecol J 33(7):2013–2020. https://doi.org/10.1007/s00192-021-05002-7
Avery K, Donovan J, Peters TJ et al (2004) ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 23(4):322–330. https://doi.org/10.1002/nau.20041
Laycock J, Jerwood D (2001) Pelvic floor muscle assessment: The PERFECT scheme. Physiotherapy 87:631–642. https://doi.org/10.1016/S0031-9406(05)61108-X
Kaya S, Akbayrak T, Toprak Çelenay Ş, et al (2015) Reliability and validity of the Turkish King’s Health Questionnaire in women with urinary incontinence. Int Urogynecol J 26(12):1853–1859. https://doi.org/10.1007/s00192-015-2786-6.
Cam C, Sancak P, Karahan N et al (2009) Validation of the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) in a Turkish population. Eur J Obstet Gynecol Reprod Biol 146(1):104–107. https://doi.org/10.1016/j.ejogrb.2009.05.016
Lagro-Janssen AL, Debruyne FM, Smits AJ, van Weel C (1992) The effects of treatment of urinary incontinence in general practice. Fam Pract 9(3):284–289. https://doi.org/10.1093/fampra/9.3.255
Yamanishi T, Yasuda K, Sakakibara R et al (2000) Randomized, double-blind study of electrical stimulation for urinary incontinence due to detrusor overactivity. Urology 55(3):353–357. https://doi.org/10.1016/S0090-4295(99)00476-8
Franzén K, Johansson JE, Lauridsen I et al (2010) Electrical stimulation compared with tolterodine for treatment of urge/urge incontinence amongst women-a randomized controlled trial. Int Urogynecol J 21(12):1517–1524. https://doi.org/10.1007/s00192-010-1213-2
Guo GY, Kang YG (2018) Effectiveness of neuromuscular electrical stimulation therapy in patients with urinary incontinence after stroke: a randomized sham controlled trial. Medicine (Baltimore) 97(52):13702. https://doi.org/10.1097/md.0000000000013702
Guo Y, Phillips BE, Atherton PJ, Piasecki M (2021) Molecular and neural adaptations to neuromuscular electrical stimulation; Implications for ageing muscle. Mech Ageing Dev 193:111402. https://doi.org/10.1016/j.mad.2020.111402
Sobhgol SS, Charandabee SM (2008) Related factors of urge, stress, mixed urinary incontinence and overactive bladder in reproductive age womenin Tabriz, Iran: a cross-sectional study. Int Urogynecol J Pelvic Floor Dysfunct 19(3):367–373. https://doi.org/10.1007/s00192-007-0437-2
McClurg D, Ashe RG, Lowe-Strong AS (2008) Neuromuscular electrical stimulation and the treatment of lower urinary tract dysfunction in multiple sclerosis-a double blind, placebo controlled, randomised clinical trial. Neurourol Urodyn 27(3):231–237. https://doi.org/10.1002/nau.20486
Ali MU, Fong KN, Kannan P et al (2022) Effects of nonsurgical, minimally or noninvasive therapies for urinary incontinence due to neurogenic bladder: a systematic review and meta-analysis. Ther Adv Chronic Dis. https://doi.org/10.1177/20406223211063059
Barroso JC, Ramos JG, Martins-Costa S et al (2004) Transvaginal electrical stimulation in the treatment of urinary incontinence. BJU Int 93(3):319–323. https://doi.org/10.1111/j.1464-410X.2004.04608.x
Verbeek M, Hayward L (2019) Pelvic floor dysfunction and its effect on quality of sexual life. Sex Med Rev 7(4):559–564. https://doi.org/10.1016/j.sxmr.2019.05.007
Su CC, Sun BY, Jiann BP (2015) Association of urinary incontinence and sexual function in women. Int J Urol 22(1):109–113. https://doi.org/10.1111/iju.12610
Dmochowski R, Lynch CM, Efros M, Cardozo L (2019) External electrical stimulation compared with intravaginal electrical stimulation for the treatment of stress urinary incontinence in women: a randomized controlled noninferiority trial. Neurourol Urodyn 38(7):1834–1843. https://doi.org/10.1002/nau.24066
Barber MD, Visco AG, Wyman JF et al (2002) Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol 99(2):281–289. https://doi.org/10.1016/S0029-7844(01)01727-6
Vase L, Petersen GL, Lund K (2014) Placebo effects in idiopathic and neuropathic pain conditions. Handb Exp Pharmacol. 225:121–136. https://doi.org/10.1007/978-3-662-44519-8_7
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). No fund was received in the present study.
Author information
Authors and Affiliations
Contributions
TB Kurt: Project development, Data Collection, Manuscript writing, Data analysis. B Yilmaz: Project development, Data Collection, Supervision. ST Celenay: Project development, Data Collection, Manuscript writing, Critical revisions to the paper. Contribution of paper. External NMES was a complementary treatment method aiming to improve urinary symptoms, PFMS, QoL, and sexual function in women with UUI. External NMES was more effective in increasing PSI and satisfaction levels in women with UUI when compared to sham NMES. Compared to internal NMES applications, external NMES applications may be preferred more in UUI treatment. There is a need for studies examining the effects of external NMES in both men and women with different pelvic floor dysfunctions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Clinical research ethics committee of Recep Tayyip Erdogan University (Approval number:2020/07).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurt, T.B., Yilmaz, B. & Celenay, S.T. Effects of external neuromuscular electrical stimulation in women with urgency urinary incontinence: a randomized sham-controlled study. World J Urol 42, 423 (2024). https://doi.org/10.1007/s00345-024-05126-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-05126-7