Abstract
Purpose
To assess the clinical performance of ProsTAV®, a blood-based test based on telomere associate variables (TAV) measurement, to support biopsy decision-making when diagnosing suspicious prostate cancer (PCa).
Methods
Preliminary data of a prospective observational pragmatic study of patients with prostate-specific antigen (PSA) levels 3–10 ng/ml and suspicious PCa. Results were combined with other clinical data, and all patients underwent prostate biopsies according to each center’s routine clinical practice, while magnetic resonance imaging (MRI) before the prostate biopsy was optional. Sensitivity, specificity, positive and negative predicted values, and subjects where biopsies could have been avoided using ProsTAV were determined.
Results
The mean age of the participants (n = 251) was 67.4 years, with a mean PSA of 5.90 ng/ml, a mean free PSA of 18.9%, and a PSA density of 0.14 ng/ml. Digital rectal examination was abnormal in 21.1% of the subjects, and according to biopsy, the prevalence of significant PCa was 47.8%. The area under the ROC curve of ProsTAV was 0.7, with a sensitivity of 0.90 (95% CI, 0.85–0.95) and specificity of 0.27 (95% CI, 0.19–0.34). The positive and negative predictive values were 0.53 (95% CI, 0.46–0.60) and 0.74 (95% CI, 0.62–0.87), respectively. ProsTAV could have reduced the biopsies performed by 27% and showed some initial evidence of a putative benefit in the diagnosis pathway combined with MRI.
Conclusions
ProsTAV increases the prediction capacity of significant PCa in patients with PSA between 3 and 10 ng/ml and could be considered a complementary tool to improve the patient diagnosis pathway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the most common neoplasia among men, except for skin cancer. An estimated 288,300 males in the US and 473,344 in Europe will receive a PCa diagnosis in 2023 [1]. Worldwide, an estimated 1.4 million people were diagnosed with PCa in 2020, making it the fourth most prevalent cancer. Since 2014, overall and advanced-stage PCa incidence rates have increased by around 3% and 5% per year, respectively [2].
Although prostate-specific antigen (PSA) is the most widely used biomarker for early diagnosis and follow-up [3], its specificity for PCa is poor, leading to unnecessary biopsies, over-diagnosis, and over-treatment [4]. European scientific societies have promoted using biomarker tests, mainly from blood, urine, imaging, or tissue-based [5]in patients with known PSA values to improve specificity and avoid over diagnosing non-significant PCa (ISUP 1) [6]. However, their clinical utility should be explored, including screening and pre-biopsy selection and comparison, or in combination with tools such as risk nomograms and magnetic resonance imaging (MRI) [7].
MRI is a valuable tool in the diagnosis of clinically significant prostate cancer (csPCa) identifying biopsy candidates [8]. However, a lack of resources in most radiology departments makes MRI difficult for many patients. In addition to the necessary training of MRI specialists, standardization of the technique is required [9] given the significant variability in scan quality and the experience and skill of the interpretation by the radiologist.
The European Commission has recommended developing prediction models of PCa based on multiple biomarkers. However, in most cases, current biomarkers lack the required validation to be implemented in routine clinical practice [10].
Specific telomere length measurements in peripheral blood leucocytes have been associated with higher PCa risk and a greater risk of aggressive disease [11]. Telomere Analysis Technology® (TAT®) combines advanced techniques to assess telomere-associated variables (TAV) in cells [12]. ProsTAV is a CE-marked in vitro diagnostic, minimally invasive, and easy-to-implement biomarker test developed by Life Length. It was developed using TAV as a complementary tool to diagnose csPCa for patients with elevated PSA (between 3 and 10 ng/ml).
A recent study showed that subjects with PSA levels between 3 and 10 ng/ml may benefit from using ProsTAV [13] demonstrating its potential to reduce the biopsies in subjects of uncertain risk by 33%. Here, we present the preliminary results of the validation extension study to determine the efficacy of ProsTAV in diagnosing csPCa.
Methods
A multicenter prospective observational study was performed on patients suspicious of PCa who underwent prostate biopsy from June 2022 to March 2023 in ten hospitals in Spain and the US. Ethical approval was granted by local committees (clinicaltrials.gov NCT04124900), and informed consent was obtained from all participants.
Population
Eligible patients were Caucasian males ≥ 18 years of age, at PCa-risk (PSA between 3 and 10 ng/ml), and biopsy candidates according to current clinical practice [5]. Patients treated with alpha-5-reductase inhibitors, with any active liver, lung, or kidney disease, severe infection, mental impairment to follow the study procedures, or other active neoplasia were excluded.
Prostate-related assays
PSA and free PSA values and digital rectal examination (DRE) were performed according to local standard practice. All patients underwent a transrectal or trans perineal prostate biopsy, systematic or with template +/- target biopsy, in case of suspicious lesion on MRI. Uropathologists from each major referral center evaluated specimens following the recommendations of the International Society of Urological Pathology (ISUP) [14]. Patients with clinically significant PCa were those with ISUP > 1 (Gleason score ≥ 7). Each participating hospital followed local MRI protocols based on PIRADS (prostate imaging reporting and data system by the International Prostate MRI Working Group) scoring 2.1 [15].
Telomere analysis technology- ProsTAV
TAT determines the telomeric length in the cell nucleus of isolated blood lymphocyte samples. It labels telomeres by using the in-situ hybridization technique (HT Q-FISH) with specific fluorescently labeled peptide nucleic acid (PNA) probes, detecting them on a high-throughput Perkin Elmer (Revvity) Phenix microscope [16]. Data were analyzed using proprietary software to generate all TAVs validated through the same CLIA and ISO15189 laboratory standards. The validation analyses were conducted on samples from the included subjects on mononuclear cells isolated from peripheral blood. These tests were performed in the laboratories of Life Length, S.L. (Madrid, Spain)
ProsTAV is a risk estimation test based on algorithm-derived models that use TAV as a biomarker diagnosing PCa (also called “risk score”) based on a refined model. Data derives from telomeric measurements and the patient’s clinical history to indicate whether the individual is at low risk and, therefore, avoid performing a prostate biopsy for the diagnoses of PCa. A biopsy is only recommended when the ProsTAV score is above 10% [13]. ProsTAV was developed by multivariate logistics regression based on clinical variables (PSA, free PSA, age, previous biopsy, and DRE) and TAV. The latter consists of three “short telomere” (percentage of telomeres of a sample with a length under a specific threshold) and three “short cell” (percentage of cells of a sample whose telomeres average a length under a specific threshold) variables [13]. The samples were handled according to ProsTAV’s technical specifications, so the cold supply chain was always maintained, and the lymphocytes were isolated within 72 h of sample extraction from the patient [17].
Statistic methods
Descriptive statistics were used to present baseline characteristics of enrolled participants, including age, PSA, free PSA, PSAD, MRI PIRADS score, and ProsTAV results. Data are expressed as means and standard deviation unless otherwise stated. All tests were considered two-tailed; p-values below 0.05 were considered statistically significant. Statistical analysis of the data was performed using SPSS® software R.8.01 and R V.4.3.1 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R). The predictive capacity and accuracy of ProsTAV were summarized by receiver operating characteristic curves (ROC) through another open-access calculator (https://datatab.es/statistics-calculator/roc-calculator).
Results
A total of 251 patients were included in this preliminary analysis. Their characteristics and csPCa results according to the ISUP grading system are shown in Table 1.
ProsTAV performance and clinical validation
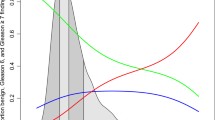
The two main values analyzed for the precision and consistency of ProsTAV, i.e., sensitivity and specificity, were 0.90 and 0.27, respectively. The positive predictive value (PPV) was 0.53, while the negative predictive value (NPV) was 0.74. When data was analyzed in the subgroup of age > 50 years and PSA > 4 ng/ml (n = 226), the sensitivity was 0.92, the specificity 0.26, NPV 77%, and false negative rate (FNR) 8%. No aggressive cancers (Gleason score ≥ 8) were missed with ProsTAV. The predictive capacity and accuracy of ProsTAV are summarized in the ROC curve (AUC = 0.7; 95% IC; 0.63–0.77) (Fig. 1).
ProsTAV likelihood ratio results
The ProsTAV positive and the negative likelihood ratios found were 1.23 and 0.37, respectively. In our sample, in those subjects with a positive ProsTAV result (> 10), the probability of csPCa was 53%. Conversely, if the patient showed a negative ProsTAV result (≤ 10), the probability of having csPCa was 25%, gaining an additional 23% of the likelihood for detection.
ProsTAV® findings versus biopsy
When comparing the results obtained by the biomarker test versus those provided by the biopsy, it was observed that ProsTAV was accurate in 143 patients (57%), 108 patients with csPCa, and 35 healthy individuals. In contrast, ProsTAV did not match the biopsy results in 12 patients with csPCa and 96 healthy individuals. Thus, ProsTAV failed to identify 10% of the csPCa (sensitivity). On the other hand, since there were 131 individuals who, according to the biopsy, did not present csPCa, and ProsTAV identified 35 of them, the biomarker test could have avoided 27% of the biopsies performed.
ProsTAV findings versus MRI
MRI was performed on 207 (82.5%) subjects (Table 1). This technique showed that 24 patients were not imaging suspicious for cancer. The positive ProsTAV result (score > 10) would have rescued 21 patients in the cohort who had negative or equivocal MRI (PIRADS ≤ 3), and biopsy proved csPCa (Fig. 2). Besides, the prediction of combining ProsTAV score and MRI in the subgroup of patients with PI-RADS ≥ 3 results in an AUC equal to 0.74 (95% CI; 0.664–0.813).
Comparison of the ProsTAV result and the PI-RADS score obtained by MRI. Patients were classified as having csPCa or non-significant PCa after biopsy. The ProsTAV score threshold is indicated as a solid line. Abbreviations: MRI, magnetic resonance imaging; PI-RADS, prostate imaging reporting, and data system; NS, non-significant PCa
The detection rate between biopsy, imaging, and ProsTAV results for those who would have been potentially selected for biopsy and those who would not are detailed in Fig. 2. According to these, MRI would have lost seven, six, and eleven (n = 24) patients with PIRADS 1, 2, and 3, respectively, from which ProsTAV would have correctly sent to biopsy seven, six, and eight (n = 21), respectively.
Discussion
This study offers external validity results for the ProsTAV biomarker test, proving that ProsTAV could have reduced the biopsies performed significantly and showed some benefit in complementing MRI in the diagnosis pathway. Our investigation confirms the clinical utility of ProsTAV in managing patients at risk for csPCa in routine clinical practice. In our investigation, 47.8% of patients had csPCa, a higher number than in previous studies of ProsTAV (19.1% in the ONCOCHECK study [12] and 23.9% in the previous algorithm validation study [13]. This is very likely since selected patients will be planned for biopsy. In fact, among MRI results, PIRADS 4 was the most frequent. The population was well distributed in different PCa stages, so adequate comparative results can be drawn. The study population was elderly, with similar suspicious DRE results to those found in the former study [13].
The sensitivity, specificity, PPV, and NPV results obtained by ProsTAV in our cohort align with those previously published [13] (identical sensitivity, specificity 0.27 and 0.33, PPV 0.53 and 0.29 and NPV 0.74 and 0.91, respectively), validating the algorithm in a clinical pragmatic cohort. In addition, the AUC provided by the ROC curve of this study is consistent with the previous study (0.7 both). Differences in the PPV and NPV data can be explained by the different PCa prevalences. Other diagnostic tests are also affected by prevalence, as observed in biomarkers MDX [18], PHI [19] PCA3 [20], or even MRI [21].
It should be noted that the clinical validation data obtained for the subgroup of men > 50 years and PSA > 4 ng/dL are better than those obtained in the overall cohort. Specifically, the analytical validity data regarding sensitivity, NPV, and FNR are slightly improved. This is relevant because this subpopulation is tested on similar biomarkers [22, 23]. It is interesting to contextualize it versus the analytical values established by other biomarkers tests marketed [24,25,26,27]. ProsTAV identified all cases of aggressive PCa in the population studied, and the missed cases, according to ProsTAV, in subjects with Gleason 7, were higher in those with the best prognosis, i.e., Gleason 7 (3 + 4). All subjects with ISUP4 and ISUP5 values showed a positive ProsTAV score (> 10). Similarly, the biomarker test accurately diagnosed csPCa in 96.4% of the individuals with ISUP2 and 98.8% of those with ISUP3.
Likelihood ratios enhance the evaluation of the clinical value of test results by integrating all the raw data [28, 29]. Our cohort established 47.8% as the odds of having csPCa, by biopsy, the gold standard technique. After using ProsTAV, this probability is adjusted. Thus, in those patients with a positive ProsTAV result, the probability of having csPCa is 53% (5% higher), indicating moderate to high efficiency of the test [30], so it can be established that a positive ProsTAV is 5.2% more likely to have csPCa than a person who has not been tested. On the other hand, a negative ProsTAV result indicates a 25% probability of csPCa. Thus, a negative ProsTAV result is 27.2% less likely to involve csPCa than a person not tested, indicating ProsTAV is an efficient test [30].
Despite the current focus on MRI, it needs to be more precise to replace biopsy, mainly because of its variable accuracy [21], so ProsTAV could contribute to decision-making. In fact, 13 patients (6.28%) with csPCa showed PIRADS 1 or 2, while the biomarker test could identify accurately through a ProsTAV score > 10.
Whether patients with a PIRADS 3 lesion require a biopsy remains controversial, PIRADS 4 or 5 lesions do so [31, 32]. These negative MRI-selected patients likely had high-risk usual variables such as elevated PSA or PSA density, a low free PSA, or a strong family history. It should be considered that ProsTAV also considers some of these biomarkers/factors. Also, in this group, ProsTAV could be a complementary tool since 24 patients (11.59%) would not have been sent to biopsy according to their PIRADS results 3 (n = 12, 5.80%) or 4 and 5 (n = 12, 5.80%). In addition, the prediction of combining ProsTAV and MRI data in patients with suspicious lesions (PI-RADS ≥ 3) results in a higher AUC value increasing the diagnostic ability (0.74 vs. 0.70).
Awaiting results in clinical practice, this preliminary analysis supports the clinical utility of ProsTAV as a biomarker test. Our cohort, with unconfirmed diagnostic suspicion, has had in ProsTAV a tool to help identify biopsy needs. This would avoid causing patients to undergo this procedure unnecessarily and save healthcare resources and costs.
There might be a selection bias of patients caused by the pragmatic cohort of already selected patients for prostate biopsy. In our study, subjects were considered at risk for PCa if PSA > 3 ng/ml and/or positive DRE. However, PCa could also be suspected with PSA values < 3 ng/ml in some countries and have not been included in the present cohort. Therefore, a lower limit of PSA needs to be studied in the future, and the biomarker’s putative role in a not previously selected PIRADS 1–3 population. Besides, variability in the biopsy methodology could be an issue, although positive from a pragmatic point of view. There is a sparse number of patients with prostatectomy specimens to be evaluated, but another study, specifically in patients with prostatectomy, to evaluate the biomarker from a prognostic perspective is planned.
To conclude, and within the scope of the clinical utility of ProsTAV as a biomarker test for PCa, the current research shows the validation of the data obtained in the study used to develop the algorithm. ProsTAV could contribute to MRI in the current diagnoses’ pathway of PCa, and this possibility needs to be further explored in future ampler prospective trials.
References
World Cancer Research Fund International Prostate cancer statistics | World Cancer Research Fund International https://www.wcrf.org/cancer-trends/prostate-cancer-statistics/. Accessed 19 Jan 2024
Cancer.Net (2023) Prostate Cancer: Statistics. https://www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed 26 Oct 2023
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E (1987) Prostate-specific Antigen as a serum marker for Adenocarcinoma of the prostate. N Engl J Med 317:909–916
Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, Carroll P, Etzioni R (2014) Overdiagnosis and overtreatment of prostate cancer. Eur Urol 65:1046–1055
European Association of Urology (2022) EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam 2022
Kim JH, Hong SK (2021) Clinical utility of current biomarkers for prostate cancer detection. Investig Clin Urol 62:1–13
Van Poppel H, Hogenhout R, Albers P, van den Bergh RCN, Barentsz JO, Roobol MJ (2021) Early detection of prostate Cancer in 2020 and Beyond: facts and recommendations for the European Union and the European Commission. Eur Urol 79:327–329
Drost F-JH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, Schoots IG (2019) Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev 4:CD012663
Giganti F, Kirkham A, Kasivisvanathan V, Papoutsaki MV, Punwani S, Emberton M, Moore CM, Allen C (2021) Understanding PI-QUAL for prostate MRI quality: a practical primer for radiologists. Insights Imaging. https://doi.org/10.1186/s13244-021-00996-6
Beyer K, Moris L, Lardas M et al (2022) Diagnostic and prognostic factors in patients with prostate cancer: a systematic review. BMJ Open 12:e058267
Julin B, Shui I, Heaphy CM, Joshu CE, Meeker AK, Giovannucci E, De Vivo I, Platz EA (2015) Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br J Cancer 112:769–776
Rubio Galisteo JM, Fernández L, Gómez Gómez E et al (2021) Telomere-based risk models for the early diagnosis of clinically significant prostate cancer. Prostate Cancer Prostatic Dis 24:88–95
Gómez Gómez E, Cano Castiñeira R, Burgos J et al (2023) ProsTAV, a novel blood-based test for biopsy decision management in significant prostate cancer. Prostate 83:1323–1331
JLH, van Leenders G, van der Kwast TH, Grignon DJ et al (2020) The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma
PI-RADS ® v2.1 PI-RADS ® Prostate Imaging-Reporting and Data System 2019 Version 2.1 PI-RADS ® Prostate Imaging-Reporting and Data System 2019 Version 2.1
Canela A, Vera E, Klatt P, Blasco MA (2007) High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci U S A 104:5300–5305
Life Length Oncology Diagnostics (2024) ProsTAV Technical Specifications Rev 1
Lendínez-Cano G, Ojeda-Claro AV, Gómez-Gómez E et al (2021) Prospective study of diagnostic accuracy in the detection of high-grade prostate cancer in biopsy-naïve patients with clinical suspicion of prostate cancer who underwent the select MDx test. Prostate 81:857–865
Furuya K, Kawahara T, Narahara M et al (2017) Measurement of serum isoform [–2]proPSA derivatives shows superior accuracy to magnetic resonance imaging in the diagnosis of prostate cancer in patients with a total prostate-specific antigen level of 2–10 ng/ml. Scand J Urol 51:251–257
Chevli KK, Duff M, Walter P, Yu C, Capuder B, Elshafei A, Malczewski S, Kattan MW, Jones JS (2014) Urinary PCA3 as a predictor of prostate Cancer in a cohort of 3,073 men undergoing initial prostate biopsy. J Urol 191:1743–1748
Moldovan PC, Van den Broeck T, Sylvester R et al (2017) What is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in excluding prostate Cancer at Biopsy? A systematic review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol 72:250–266
Prostate Health Index (phi) | Beckman Coulter. https://www.beckmancoulter.com/es/products/immunoassay/phi. Accessed 20 Nov 2023
IsoPSA® TESTING | Cleveland Diagnostics — Cleveland Diagnostics. https://www.clevelanddx.com/isopsa-testing. Accessed 20 Nov 2023
Gen-Probe (2012) Gen-Probe Incorporated, ‘Physician Brochure for the PROGENSA ® PCA3 Assay
Haese A, Trooskens G, Steyaert S et al (2019) Multicenter optimization and validation of a 2-Gene mRNA urine test for detection of clinically significant prostate Cancer before initial prostate biopsy. J Urol 202:256–263
Food and Drug Administration (FDA) (2012) PHI SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED)
U.S. Food and Drug Administration (2021) 4Kscore SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED)
Radack KL, Rouan G, Hedges J (1986) The likelihood ratio. An improved measure for reporting and evaluating diagnostic test results. Arch Pathol Lab Med 110:689–693
Likelihood Ratios — Centre for Evidence-Based Medicine (CEBM) University of Oxford. https://www.cebm.ox.ac.uk/resources/ebm-tools/likelihood-ratios. Accessed 3 Nov 2023
Riewe E, Neubauer E, Pfeifer AC, Schiltenwolf M (2016) Predicting persistent back symptoms by psychosocial risk factors: validity criteria for the ömpsq and the hkf-r 10 in Germany. PLoS ONE. https://doi.org/10.1371/journal.pone.0158850
Schoots IG (2018) MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions? Transl Androl Urol 7:702–782
Kaufmann B, Saba K, Schmidli TS et al (2021) Prostate cancer detection rate in men undergoing transperineal template-guided saturation and targeted prostate biopsy. https://doi.org/10.1002/pros.24286. Prostate
Acknowledgements
The authors thank Enrique Segovia and Nuria de Pedro (Life Length S. L.) for collaborating on this study. María Romero Olleros (Trialance SCCL) provided medical writing support in the development of this article.
Funding
This study was funded by Life Length S.L.
Author information
Authors and Affiliations
Contributions
MPP and LF: project development. AM, PH and MPP: responsible for data collection. EGG, JIM-S, FB, BJM, JB, RCC, ARA: Data collection. MPP: manuscript drafting. All authors: revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
LF, AM, PH and MP are current employees of Life Length. EGG, JIM-S, FB, BJM, JB, JJQ, RCC, ÁG-F, ARA, GC were recipients of a promotor (Life Length, S.L) grant for patient inclusion. All other authors report no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gómez-Gómez, E., Martínez-Salamanca, J.I., Bianco, F. et al. ProsTAV, a clinically useful test in prostate cancer: an extension study. World J Urol 42, 395 (2024). https://doi.org/10.1007/s00345-024-05098-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-05098-8