Abstract
Purpose
Despite many efforts, no reliable urinary marker system has so far shown the potential to substitute cystoscopy. Measuring volatile organic compounds (VOCs) from urine is a promising alternative. VOCs are metabolic products which can be measured from the headspace of urine samples. Previous studies confirmed that the urine of bladder tumor patients has a different VOC profile than healthy controls. In this pilot study, the feasibility of discriminating VOCs from urine of bladder cancer patients from that of healthy control subjects was investigated. Aim of this study was to investigate whether VOC-based diagnosis of bladder cancer from urine samples is feasible using multicapillary column ion mobility spectrometry (MCC/IMS) and to identify potential molecular correlates to the relevant analytes.
Methods
Headspace measurements of urine samples of 30 patients with confirmed transitional cell carcinoma (TCC) and 30 healthy controls were performed using MCC/IMS. In the results of the measurements, peaks showing significant differences between both groups were identified and implemented into a decision tree with respect to achieve group separation. Molecular correlates were predicted using a pre-defined dataset.
Results
Eight peaks with significantly differing intensity were identified, 5 of which were highly significant. Using a six-step decision tree, MCC/IMS showed a sensitivity of 90% and specificity of 100% in group separation.
Conclusion
VOC-based detection of bladder cancer is feasible. MCC/IMS is a suitable method for urine-based diagnosis and should be further validated. The molecular characteristics and metabolic background of the analytes require further workup.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer has been showing a slow rise in incidence over the past years. In Germany, there were a total of 31,040 cases in 2018; one third of those were non-invasive pTa tumours [1]. 80% of bladder cancer patients present with haematuria which accounts for the fact that most patients are diagnosed at an early stage [2]. Hence, all patients with visible haematuria should undergo a cystoscopy which will be positive in 20% of cases. Cystoscopy is also required during the follow-up of non-muscle invasive bladder cancer as there is a significant risk of recurrence.
Many attempts have been made to evade cystoscopy as it is not only unpopular with patients due to its invasive and uncomfortable nature but also time-consuming and expensive. So far, no urine-based biomarker system other than cytology has successfully been implemented into clinical routine and guidelines [3]. The measurement of volatile organic compounds (VOC) could be a promising alternative to conventional urinary biomarkers. VOCs are metabolic products which are emitted via breath, urine, and other body secretions. Many diseases lead to characteristic alterations in VOC profiles which can be used for diagnostic purposes. VOC detection has been shown to be effective in the diagnosis of a range of pulmonary, neurological, and malignant disease from breath as well as inflammatory bowel disease from stool [4,5,6,7,8,9,10]. Our group previously reported a pilot study on the VOC-based detection of bladder cancer from the headspace of urine samples using an electronic nose [11]. Electronic noses use pattern recognition to allocate gas samples to groups. Measurements are fast and can be conducted at the bedside. However, those systems do not measure individual molecular components of the gas sample. Therefore, an identification of substances as potential targeted biomarkers is not possible. Ion mobility spectrometry (IMS) is a semi-quantitative method which can differentiate between groups and enables the identification of individual components of a mixed gas sample (using a reference dataset).
The aim of this study was to establish the method of headspace measurements independent from ambient air for measuring and distinguishing urine samples and to create specific VOC profiles using IMS as a novel non-invasive, cost-effective, and precise method to detect bladder cancer.
The primary objective was to evaluate whether IMS could detect differences between the smell print of urine samples of bladder cancer patients and healthy controls. Secondary objectives were to investigate whether IMS was more accurate than measurements using electronic noses and to identify individual molecular substances specific to bladder cancer which could potentially be used as biomarkers in the future.
Materials and methods
Patients and sample storage
Fourty-two patients with cystoscopically confirmed bladder tumors referred for TUR-BT were recruited on the day of their pre-assessment. Patients with urinary tract infection on urine dipstick, indwelling bladder catheters or ureteric stents, and patients with known other malignancies were excluded. Twelve patients were excluded as no malignancy was found on histopathological workup. Thirty individuals with no known disease of the urinary tract (including urinary tract infections) were recruited as healthy controls. Samples of first void morning urine were collected for analysis and stored in 2 ml vials at −20 °C until measurement.
Ethics approval for this study was given by the local ethics committee (Az 131/14). All participants were informed about the study and handed an information sheet; written consent was obtained.
Headspace measurements
Samples were thawed at room temperature and then vortexed and heated to 37 °C in a water bath for 10 min before being transferred into a sealable vial that could be connected to the tubing system of the IMS. We used a BioScout IMS (B and S Analytik GmbH, Dortmund, Germany) combined with an up-streamed multicapillary column (MCC, type OV-5, Multichrom Ltd, Novosibirsk, Russia) to pre-grade volatile analytes. Gaseous material arising from the heated urine samples was exposed and sucked into the IMS for measurement. A standardised flushing procedure to clean the sensors was performed according to the manufacturer’s recommendations after each measurement.
Analysis of MCC-IMS data
Raw data from IMS measurements was analyzed using the software Visual Now 2.2 (B and S Analytik GmbH, Dortmund, Germany). The electronic signals caused by single ion impact were visualized as peaks, the height of which being characterized by ion concentration and its position with regard to drift time and retention time. Hence, every sample running through the spectrometer created a unique set of peaks. Based on the database 20160426_SubstanzDbNIST (B and S Analytik GmbH, Dortmund, Germany), the peaks were compared to database-derived characteristics of known substances using MIMA, a software for VOC detection. Rapid Miner 7.5 (Rapid Miner GmbH, Boston, MA, USA) was used to generate a multi-level decision tree to differentiate between groups of samples.
The team in charge of IMS data analysis was blinded to the results of the pathology reports and vice versa.
Statistics and data analysis
All IMS analyses were calculated with SPSS 22 (IBM SPSS Statistics, Version 22.0. Armonk, New York, USA) and Prism 5.03 (GraphPad Software, Inc., La Jolla, USA). Mann–Whitney-U-Test for unpaired samples was used to compare between two groups while for within-group comparisons, a Wilcoxon rank-sum Test for paired samples was performed. For comparison of measurements from several time-points, Friedman’s test for continuous and Fisher’s exact test for categorical variables were performed. All tests were two-sided (p < 0.05 was considered to be significant). For independent samples, the Kruskal–Wallis test was used. For patient and tumor characteristics, categorical variables were evaluated as counts and percentages, and chi-square test was used; continuous variables were evaluated by using mean ± standard deviation; parametric variables were evaluated with t-test.
Results
The demographics of study participants are summarized in Table 1.
Histopathology confirmed urothelial carcinoma in 30 patients from the tumor group with a representative range of tumor stages. Tumor characteristics are shown in Table 2.
A total of 82 peaks representing individual molecular components of the gas samples were identified. Of those, 8 peaks showed significantly different levels of signal intensity between groups with p < 0.01 and 5 peaks were highly significant with p < 0.001.
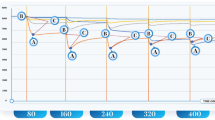
As an example, Fig. 1 shows the box whisker plot for peak P29 after Bonferroni correction.
In order to differentiate between tumor and control samples, a decision tree based on significant peaks was generated using six steps (Fig. 2).
This resulted in a sensitivity of 90% and a specificity of 100%. Further statistics of the decision tree are shown in Table 3.
The peaks which were used in this model were compared with the aforementioned database in order to identify potential molecular correlates based on previous IMS experiences followed by in-depth molecular characterization of analytes. The following possible correlations were established: P29: Benzylaldehyde (dimer) or benzofurane; JI36: ammonia; P8: toluol; JI19: hexylbenzene; P13: cyclohexene 1-methyl-4-(1-methylethylidene); P28: acetyl valeryl.
No significant differences in measurement patterns could be identified between high grade and low grade tumors or for different T stages or for recurrent versus primary disease. Results for smokers and non-smokers did not differ. The presence of microscopic hematuria did not result in significantly different results either.
Discussion
In this pilot study, small cohorts of confirmed bladder cancer patients and healthy controls were compared. Using MCC/IMS, it could be shown that the two groups have different VOC profiles and the peaks relevant for group separation were identified.
Using a decision tree-based model, IMS could separate between urine samples of bladder cancer patients and healthy controls with a sensitivity of 90% and specificity of 100%. Potential molecular correlates to the most relevant individual gaseous analytes were suggested.
Little research has been done so far on VOC-based diagnosis of bladder cancer. While only using a comparatively small sample size and a highly standardized setting, our study provides further evidence that bladder cancer diagnosis based on VOC analysis is feasible in principle. They confirm our previous findings with the electronic nose Cyranose 320™ which showed a sensitivity of 93.3% and specificity of 87.7% [11].
Other groups found similar results with different VOC-based setups for bladder cancer diagnosis [12,13,14,15,16]. While those high values for sensitivity and specificity are promising and warrant further investigations of the method, the potential influence of confounders such as urinary tract infections or hematuria remains to be established in larger studies. At present, we did not find any differences in the measurement patterns of patients with or without microscopic hematuria. However, this might be an effect of the small sample size in this pilot study.
A follow-up study validating the method in patients presenting for hematuria workup is currently underway at our center. Another aspect to be looked at in future research is whether VOCs can help to differentiate between high and low grade tumors or T stages. In this limited data set, we did not find significant differences.
In comparison to VOC-based methods, the only urine-based diagnostic method recommended for bladder cancer by various guidelines — nurine cytology — has a sensitivity of 34% and specificity of 99% [17]. A plenitude of urine-based marker systems has been introduced over the years but none was convincing enough to be implemented into clinical routine. The most relevant were summarized in a systematic review by Ng in 2021 [3]. NMP22 demonstrated a sensitivity of 52–59% and a specificity of 87–89%. The BTA Stat test performed at a sensitivity of 57–82% and specificity of 68–93%, and FISH-based UroVysion showed a sensitivity of 69–87% and specificity of 89–96%. Multigene panels and miRNA showed potentially better results depending on the approach but are more expensive and time-consuming. Hence, pattern recognition based on VOCs which also uses a multi-target approach seems to be a more sensible approach than conventional biomarkers. Cystoscopy itself, which remains the golden standard, was found to have a sensitivity of 62–84% and specificity of 43–93%. When using photodynamic diagnostics, this improved the sensitivity to 82–97% at a specificity of 35–98% [18]. Keeping that in mind, a non-invasive option sparing patients from cystoscopy seems not too far away.
Within the range of VOC detection methods, MCC/IMS represents a compromise between rapid and easy to perform approaches solely based on pattern recognition such as electronic noses and most accurate but slow and expensive methods allowing for single analyte characterization such as gas chromatography/mass spectrometry (GC/MS). One advantage is that it allows to obtain time serial measurements enabling insight into the dynamics of VOC release. The current IMS applications available require manual analysis, but for different indications, bedside variations have already been introduced since 2017 [19].
A strength of this study is its simple design with direct comparison of tumor patients and benign control aiming for optimal group separation. The patient cohort was representative for bladder cancer patients in a real-world setting. One of its limitations clearly is the comparatively small sample size.
The mean age between patients in the two groups differed significantly. Given our experience from previous studies, it is unlikely but conceivable that this factor would influence the VOC profile.
The findings have yet to be validated in a cohort at risk such as patients with visible hematuria undergoing a workup for bladder cancer. This is being investigated in the aforementioned follow-up study. It remains to be seen whether the very promising sensitivity and specificity can be reproduced in a non-filtered cohort.
Last but not least, the analytes that we suspect behind the most significant measurement peaks may or may not be correctly predicted and require further analysis with GC/MS. The underlying metabolic pathways are still unclear.
These should be the next steps in implementing the VOC-based approach. Nevertheless, there is evidence that single analyte marker systems for bladder cancer are inferior to multi-target setups. Thus, pattern recognition may prove to be more sensible than developing a targeted measurement system for individual analytes identified by MCC/IMS or GC/MS.
MCC/IMS as a technology is promising and reliable but so far, it is solely an exploratory method which requires expert data handling with time-consuming manual peak picking, hence it is not a good candidate for clinical routine in the current form. However, there is already work being done in order to implement artificial intelligence algorithms to significantly simplify data analysis.
With regard to a future transition of VOC detection from research to a clinical routine laboratory, MCC/IMS and GC/MS findings may also help to identify the relevant panel of molecular components that are responsible for the smell print of bladder cancer in urine. Those components could then be included in a purpose-designed electronic nose which would enable rapid bedside testing. This approach of modified “nose chips” is already being practiced by manufacturing companies in the field of airport and military security.
Whether VOC-based methods have a role in follow-up for NMIBC and in the detection of upper urinary tract urothelial cancer is yet to be determined.
In conclusion, the detection of volatile organic compounds is a promising approach for reliable non-invasive diagnosis and potentially for the follow-up of bladder tumors. While the current clinical significance of the method is limited, it warrants further validation studies with larger sample sizes.
Data availability
All relevant data supporting the findings of this study are available within the paper. Raw measurement data from MCC/IMS of individual samples are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Philipps-Universität Marburg.
References
Krebs in Deutschland [Cancer in Germany] 2017/2018: Robert-Koch-Institut; 2021
Edwards TJ, Dickinson AJ, Natale S, Gosling J, McGrath JS (2006) A prospective analysis of the diagnostic yield resulting from the attendance of 4020 patients at a protocol-driven haematuria clinic. BJU Int 97(2):301–305
Ng K, Stenzl A, Sharma A, Vasdev N (2021) Urinary biomarkers in bladder cancer: a review of the current landscape and future directions. Urol Oncol 39(1):41–51
Hattesohl AD, Jorres RA, Dressel H, Schmid S, Vogelmeier C, Greulich T et al (2011) Discrimination between COPD patients with and without alpha 1-antitrypsin deficiency using an electronic nose. Respirology 16(8):1258–1264
Greulich T, Hattesohl A, Grabisch A, Koepke J, Schmid S, Noeske S et al (2013) Detection of obstructive sleep apnoea by an electronic nose. Eur Respir J 42(1):145–155
de Heer K, Kok MG, Fens N, Weersink EJ, Zwinderman AH, van der Schee MP et al (2016) Detection of airway colonization by aspergillus fumigatus by use of electronic nose technology in patients with cystic fibrosis. J Clin Microbiol 54(3):569–575
Bach JP, Gold M, Mengel D, Hattesohl A, Lubbe D, Schmid S et al (2015) Measuring compounds in exhaled air to detect Alzheimer’s disease and Parkinson’s disease. PLoS ONE 10(7):e0132227
Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A et al (2010) Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer 103(4):542–551
de Meij TG, de Boer NK, Benninga MA, Lentferink YE, de Groot EF, van de Velde ME et al (2014) Faecal gas analysis by electronic nose as novel, non-invasive method for assessment of active and quiescent paediatric inflammatory bowel disease: proof of principle study. J Crohns Colitis. https://doi.org/10.1016/j.crohns.2014.09.004
Probert CS, Reade S, Ahmed I (2014) Fecal volatile organic compounds: a novel, cheaper method of diagnosing inflammatory bowel disease? Expert Rev Clin Immunol 10(9):1129–1131
Heers H, Gut JM, Hegele A, Hofmann R, Boeselt T, Hattesohl A et al (2018) Non-invasive detection of bladder tumors through volatile organic compounds: a pilot study with an electronic nose. Anticancer Res 38(2):833–837
Zhu S, Corsetti S, Wang Q, Li C, Huang Z, Nabi G (2019) Optical sensory arrays for the detection of urinary bladder cancer-related volatile organic compounds. J Biophotonics 12(10):e201800165
Lett L, George M, Slater R, De Lacy CB, Ratcliffe N, Garcia-Finana M et al (2022) Investigation of urinary volatile organic compounds as novel diagnostic and surveillance biomarkers of bladder cancer. Br J Cancer 127(2):329–336
Khalid T, White P, De Lacy CB, Persad R, Ewen R, Johnson E et al (2013) A pilot study combining a GC-sensor device with a statistical model for the identification of bladder cancer from urine headspace. PLoS ONE 8(7):e69602
Zhu S, Huang Z, Nabi G (2020) Fluorometric optical sensor arrays for the detection of urinary bladder cancer specific volatile organic compounds in the urine of patients with frank hematuria: a prospective case-control study. Biomed Opt Express 11(2):1175–1185
Weber CM, Cauchi M, Patel M, Bessant C, Turner C, Britton LE et al (2011) Evaluation of a gas sensor array and pattern recognition for the identification of bladder cancer from urine headspace. Analyst 136(2):359–364
Lotan Y, Roehrborn CG (2003) Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology 61(1):109–118
Jocham D, Stepp H, Waidelich R (2008) Photodynamic diagnosis in urology: state-of-the-art. Eur Urol 53(6):1138–1148
Bous M, Tutdibi E, Nourkami-Tutdibi N, Kaiser E, Stutz R, Meyer S et al (2023) Patterns of volatile organic compounds in excrements of preterm neonates. Eur J Clin Invest 53(1):e13868
Acknowledgements
The Authors would like to acknowledge the support of Ursula Boas, Ecatarina Oplesch, and the late Helga Kirchner in the process of sample handling and measurements.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The study was conceived of by H Heers, R Hofmann, T Boeselt, and AR Koczulla. IMS measurements were performed and analysed by JM Gut. Statistical analysis was performed by JI Baumbach and JM Gut. Data interpretation was performed by JM Gut, T Boeselt, and H Heers. The manuscript was prepared by H Heers and critically revised by all authors. All authors had access to the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose with regard to this study.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Philipps-Universität Marburg (Az 131/14).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heers, H., Gut, J.M., Hofmann, R. et al. Pilot study for bladder cancer detection with volatile organic compounds using ion mobility spectrometry: a novel urine-based approach. World J Urol 42, 353 (2024). https://doi.org/10.1007/s00345-024-05047-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-05047-5