Abstract
Background
Τhe adherence of p-fimbriated Escherichia coli (E. coli) to urothelial cells leading to recurrent urinary tract infections (rUTIs) may be prevented by proanthocyanidins (PACs) contained in American cranberries.
Purpose
The purpose of this clinical trial was to assess the clinical utility of prophylactic use of high-dose PACs daily in women with a history of rUTIs.
Materials and methods
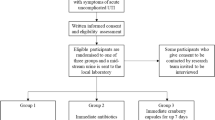
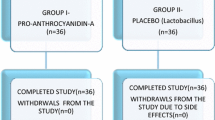
172 adult women with a history of rUTIs, defined as ≥ 2 within a 6-month period or ≥ 3 within a 12-month period were enrolled and randomized in two groups to receive either Cysticlean™ 240 mg or placebo for a 12-month period. Urine samples, vaginal and rectal swabs were collected at initial and quarterly study visits. The primary study endpoints were the number of urinary tract infections (UTIs) and changes in Quality of Life (QoL), assessed by the 36-Item Short Form Survey (SF-36) questionnaire.
Results
160 adult women of median age 40 years old (range 19–82) were finally analyzed in this randomized, placebo-controlled, double-blinded clinical trial. In response to intervention, the number of UTIs was significantly lower (Incidence rate ratio IRR 0.49, p < 0.001) and QoL was slightly improved. The numbers of E. coli isolates detected in vaginal (IRR 0.71, p value < 0.001) and in rectal swabs (IRR 0.87, p value < 0.001) were also significantly decreased. No adverse events were reported.
Conclusion
The daily use of Cysticlean™ 240 mg was associated with a reduction of UTIs and a prolongation of UTI-free survival compared to placebo treatment, supporting its use as prophylaxis in this patient population.
Trial registration
Clinicaltrials.gov, identifier NCT03032003.

Similar content being viewed by others
Data availability
The data presented in this study are available within the article and are kept at the Department of Hygiene and Epidemiology of Larissa in Thessaly.
References
Addis T, Mekonnen Y, Ayenew Z, Fentaw S, Biazin H (2021) Bacterial uropathogens and burden of antimicrobial resistance pattern in urine specimens referred to Ethiopian Public Health Institute. PLoS ONE 16(11):e0259602
Foxman B (2002) Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113(1):5–13
Williams G, Hahn D, Stephens JH, Craig JC, Hodson EM (2023) Cranberries for preventing urinary tract infections. Coch Data Syst Rev 4(4):CD001321. https://doi.org/10.1002/14651858.CD001321.pub6
Nemzer BV, Al-Taher F, Yashin A, Revelsky I, Yashin Y (2022) Cranberry: Chemical composition, antioxidant activity and impact on human health: Overview. Molecules 27(5):1503
Risco E, Miguélez C, Sanchez de Badajoz E, Rouseaud A (2010) Effect of American cranberry (Cysticlean) on Escherichia coli adherence to bladder epithelial cells. In vitro and in vivo study. Arch Españ Urol 63(6):422–430
González de Llano D, Moreno-Arribas MV, Bartolomé B (2020) Cranberry polyphenols and prevention against urinary tract infections: relevant considerations. Molecules 25(15):3523
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 1:473–483
Free online SF-36 score calculator – OrthoToolKit [Internet]. Available from: https://orthotoolkit.com/sf-36/
Cai T, Koves B, Johansen TE (2017) Asymptomatic bacteriuria, to screen or not to screen–and when to treat? Curr Opin Urol 27(2):107–111
Butcher NJ, Monsour A, Mew EJ, Chan AW, Moher D, Mayo-Wilson E, Terwee CB, Chee-A-Tow A, Baba A, Gavin F, Grimshaw JM (2022) Guidelines for reporting outcomes in trial reports: the CONSORT-Outcomes 2022 extension. JAMA 328(22):2252–2264
Team RD. R (2010) A language and environment for statistical computing
Babar A, Moore L, Leblanc V, Dudonné S, Desjardins Y, Lemieux S, Bochard V, Guyonnet D, Dodin S (2021) High dose versus low dose standardized cranberry proanthocyanidin extract for the prevention of recurrent urinary tract infection in healthy women: a double-blind randomized controlled trial. BMC Urol 21:1–3
Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B (2011) Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis 52(1):23–30
Occhipinti A, Germano A, Maffei ME (2016) Prevention of urinary tract infection with Oximacro®, a cranberry extract with a high content of a-type proanthocyanidins: A pre-clinical double-blind controlled study. Urol J 13(2):2640–2649
Blanco JJ, Gonzalez GT, Villa AG, del Cañizo AA, Núñez JF (2018) Uncomplicated cystitis treated with high proanthocyanidins cranberry concentration in patients aged 70 years old and below with recurrent urinary tract infections. Rev Electrón Biomed 2:9–21
Núñez JF, Hernández CP, Blanco JC, Gonzalez GT, Villa AG, Carlos AD, Musso G (2021) Cranberry dosed extract: An effective therapy for recurrent Escherichia coli cystitis in elderly patients. The GerHogar Cysticlean® study. Rev Colomb Nefrol 8:1
Stapleton AE, Dziura J, Hooton TM, Cox ME, Yarova-Yarovaya Y, Chen S, Gupta K. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. InMayo Clinic Proceedings 2012 Feb 1 (Vol. 87, No. 2, pp. 143–150). Elsevier
Sánchez Ballester F, Ruiz Vidal V, López Alcina E, Domenech Perez C, Escudero Fontano E, Oltra Benavent AM, Montoliu García A, Sobrón Bustamante MA (2013) Cysticlean® a highly pac standardized content in the prevention of recurrent urinary tract infections: an observational, prospective cohort study. BMC Urol 13:1–6
Harris E (2023) Updated Meta-analysis: Cranberry Products Reduced UTI Risk. JAMA 329(20):1730
Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE (2000) Risk factors for recurrent urinary tract infection in young women. J Infect Dis 182(4):1177–1182
Mellen CK, Ford M, Rindone JP (2010) Effect of high-dose cranberry juice on the pharmacodynamics of warfarin in patients. Br J Clin Pharmacol 70(1):139–142
Fu Z, Liska D, Talan D, Chung M (2017) Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: a systematic review and meta-analysis. J Nutr 147(12):2282–2288
Acknowledgements
Authors thank all clinical and laboratory staff involved and all participants of the study. Cysticlean™ 240mg was supplied by Stilvi Pharmaceuticals SA (Vita Green’s local representative in Greece).
Funding
The implementation of the doctoral thesis was co-financed by Greece and the European Union (European Social Fund-ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the Act “Enhancing Human Resources Research Potential by undertaking a Doctoral Research” Sub-action 2: IKY Scholarship Programme for PhD candidates in the Greek Universities.
Author information
Authors and Affiliations
Contributions
Conceptualization—protocol development, V.T., S.G. H.C.; supervision, V.T.; writing—original draft preparation, Ε.Τ., V.T., P.J.V.; writing—review and editing E.T., V.T., O.K.G., S.G., H.C., M.T., M.K., K.D., M.G.; data collection, E.T.; data analysis, M.K., A.M., K.K., E.V., statistical analysis, K.D.; project administration, V.T., S.G., H.G., E.T., and K.G.O. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki and its later amendments. The protocol, the consent form and all procedures of this trial were reviewed and approved by the institutional ethics committee of University General Hospital of Larissa (No 2017–5506/19–10-2017). Written informed consent was obtained from all study participants prior to randomization. This randomized clinical trial is registered at ClinicalTrials.gov, identifier: NCT03032003.
Consent for publication
All authors approved the final version of the manuscript for submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsiakoulias, E., Gravas, S., Hadjichristodoulou, C. et al. Randomized, placebo-controlled, double-blinded study of prophylactic cranberries use in women with recurrent uncomplicated cystitis. World J Urol 42, 27 (2024). https://doi.org/10.1007/s00345-023-04741-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-023-04741-0