Abstract
Purpose
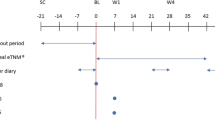
The aim of this prospective 12-month follow-up study is to evaluate the persistence of the treatment effect achieved during the initial course of peroneal electrical Transcutaneous NeuroModulation (peroneal eTNM®) in patients with overactive bladder (OAB).
Methods
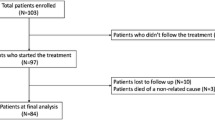
This study enrolled 21 female patients who participated in two previous clinical studies designed to assess the efficacy and safety of peroneal eTNM®. The patients were left without subsequent OAB treatment and were invited to attend regular follow-up visits every 3 months. The patient’s request for additional treatment was considered an indicator of the withdrawal of the treatment effect of the initial course of peroneal eTNM®. The primary objective was the proportion of patients with persisting treatment effect at follow-up visit 12 months after initial course of peroneal eTNM®. Descriptive statistics are presented using median, correlation analyses were computed using a nonparametric Spearman correlation.
Results
The proportion of patients with persistent therapeutic effect of the initial course of peroneal eTNM® was 76%, 76%, 62% and 48% at 3, 6, 9 and 12 months, respectively. There was a significant correlation between patient reported outcomes and the number of severe urgency episodes with or without urgency incontinence as reported by patients at each follow-up visit (p = 0.0017).
Conclusion
The treatment effect achieved during the initial phase of peroneal eTNM® persists for at least 12 months in 48% of patients. It is likely that the duration of effects is dependent on the length of the initial therapy.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Huang J, Fan Y, Zhao K et al (2002) Comparative efficacy of neuromodulation technologies for overactive bladder in adults: a network meta-analysis of randomized controlled trials. Neuromodulation S1094–7159(22):00752–00758
Sirls ER, Killinger KA, Boura JA, Peters KM (2018) Percutaneous tibial nerve stimulation in the office setting: real-world experience of over 100 patients. Urology 113:34–39
Du C, Berg W, Siegal AR et al (2021) Real-world compliance with percutaneous tibial nerve stimulation maintenance therapy in an American population. Urology 153:119–123
Del Río-Gonzalez S, Aragon IM, Castillo E et al (2017) Percutaneous tibial nerve stimulation therapy for overactive bladder syndrome: clinical effectiveness, urodynamic, and durability evaluation. Urology 108:52–58
Krhut J, Rejchrt M, Slovak M, Dvorak RV, Peter L, Blok BFM, Zvara P (2023) Prospective, randomized, multicenter trial of peroneal electrical transcutaneous neuromodulation versus solifenacin in treatment of naïve patients with overactive bladder. J Urol 209(4):734–741
Krhut J, Rejchrt M, Slovak M, Dvorak RV, Grepl M, Zvara P (2022) Peroneal electrical transcutaneous neuromodulation in the home treatment of the refractory overactive bladder. Int Urogynecol J. https://doi.org/10.1007/s00192-022-05359-3
Krhut J, Peter L, Rejchrt M, Slovak M, Skugarevska B, Zvara P (2021) Peroneal electric transcutaneous neuromodulation (eTNM®): a novel method for the treatment of the overactive bladder. J Healthc Eng 2021:4016346
Notte SM, Marshall TS, Lee M et al (2012) Content validity and test-retest reliability of Patient Perception of Intensity of Urgency Scale (PPIUS) for overactive bladder. BMC Urol 12:26
Meadows KA (2011) Patient-reported outcome measures: an overview. Br J Community Nurs 16(3):146–151
Agarwal A, Eryuzlu LN, Cartwright R et al (2014) What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. Eur Urol 65:1211–1217
Ghavidel-Sardsahra A, Ghojazadeh M, Rahnama’I MS et al (2022) Efficacy of percutaneous and transcutaneous posterior tibial nerve stimulation on idiopathic overactive bladder and interstitial cystitis/painful bladder syndrome: a systematic review and meta-analysis. Neurourol Urodyn 41:539–551
Andersen K, Kobberø H, Pedersen TB, Poulsen MH (2021) Percutaneous tibial nerve stimulation for idiopathic and neurogenic overactive bladder dysfunction: a four-year follow-up single-centre experience. Scand J Urol 55(2):169–176
Finazzi Agrò E, Campagna A, Sciobica F et al (2005) Posterior tibial nerve stimulation: is the once-a-week protocol the best option? Minerva Urol Nefrol 57:119–123
Yoong W, Ridout AE, Damodaram M, Dadswell R (2010) Neuromodulative treatment with percutaneous tibial nerve stimulation for intractable detrusor instability: outcomes following a shortened 6-week protocol. BJU Int 106:1673–1676
Jung CE, Menefee SA, Diwadkar GB (2020) 8 versus 12 weeks of percutaneous tibial nerve stimulation and response predictors for overactive bladder. Int Urogynecol J 31:905–914
Krhut J, Tintěra J, Rejchrt M et al (2023) Brain response induced by peroneal electrical transcutaneous neuromodulation invented for overactive bladder treatment, as detected by functional magnetic resonance imaging. Neuromodulation. https://doi.org/10.1016/j.neurom.2022.11.016
Blok BF, Groen J, Bosch JL, Veltman DJ, Lammertsma AA (2006) Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int 98:1238–1243
Bianchi D, Iacovelli V, Parisi I et al (2021) Real-life data on long-term follow-up of patients successfully treated with percutaneous tibial nerve stimulation. Minerva Urol Nephrol 73:260–264
Acknowledgements
The authors are grateful to L. Horčička and L. Slobodník for their contribution to the data acquisition.
Funding
The primary trials were funded by STIMVIA™, Ostrava, Czech Republic. The present follow-up study did not receive any funding from public or private sources.
Author information
Authors and Affiliations
Contributions
MR: contributed to study concept, data acquisition, data analysis, and manuscript revision; JK: contributed to study concept, study design, data acquisition, data analysis, interpretation of data, and manuscript writing; MG: contributed to study concept, data acquisition, and manuscript revision; BFMB: contributed to study concept, study design, data analysis, interpretation of data, manuscript revision, and supervision; PZ: contributed to study design, data analysis, interpretation of data, manuscript writing, manuscript editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
Michal Rejchrt is an investigator for STIMVIA; Jan Krhut is a consultant and investigator for STIMVIA; Marcel Gärtner an investigator for STIMVIA; Bertil Blok is a member of the Scientific Advisory Board at STIMVIA; Peter Zvara has no conflicts of interest to declare.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
The study was conducted in accordance with the principles of the Declaration of Helsinki, World Medical Association. An independent institutional ethics committee of Příbram Regional Hospital approved this study protocol, data collection plan, and ICF (Nr.PB205/21). The primary trials were registered under ClinicalTrial.gov nr. 05211193 and EudraCT nr. 2019-003321-14.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rejchrt, M., Krhut, J., Gärtner, M. et al. Effect duration of the initial course of peroneal electrical Transcutaneous NeuroModulation in patients with overactive bladder. World J Urol 41, 1629–1634 (2023). https://doi.org/10.1007/s00345-023-04394-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04394-z