Abstract
Purpose
To assess the benefits and risks associated with empiric prescription of antibiotic therapy for treatment of a urinary tract infection (UTI).
Methods
Following IRB approval menopausal women presenting with a symptomatic UTI to a single urology clinic were prospectively assigned to one of the two treatment groups based on day of presentation: culture-based treatment (CB) (Monday, Tuesday, Wednesday) or empiric treatment (ET) (Thursday, Friday) and started on nitrofurantoin (NF) pending culture results. Both groups were contacted at 7 and 14 days following treatment. Side effects and answers to a standardized questionnaire (UTISA) were recorded. Success was defined as a total UTISA score < 3. Any NF retreatment, use of another antibiotic therapy, or extension of the original antibiotic course was considered treatment failures.
Results
From July 2020 to March 2022, 65 women with 80 UTI events were included in the study, with CB treatment used for 60 UTIs and ET used for 23 UTIs. At 7 days after start of treatment, questionnaire failure rate was 44% (20/45) for the CB group and 16% (3/19) for the ET group (P = 0.076). At 14 days following start of treatment, questionnaire failure rate was 31% (13/42) for the CB group and 17% (3/18) for the ET group (P = 0.3). In the ET group, 11% of cultures were found to be resistant to NF.
Conclusion
Outcomes for the empiric treatment of uncomplicated UTI with NF at both 7 and 14 days are not significantly different than outcomes with culture-based treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infections (UTIs) are the most common infection leading to an antibiotic prescription after a physician’s visit in the USA [1]. Approximately 60% of women will experience symptomatic acute bacterial cystitis during their lifetime and approximately 20–40% of women who have had one UTI will have at least 1 recurrent UTI [2]. Despite published guidelines for the optimal treatment of UTIs in women, review of the literature reveals a wide variation in the prescribing practices of providers [3, 4]. While the current guidelines from the Infectious Diseases Society of America (IDSA) for the treatment of uncomplicated cystitis offer guidance on what antibiotics to use empirically, they do not specifically discuss when or whether empiric therapy should be initiated [5]. The Canadian guidelines do recommend delaying antimicrobial therapy pending urine culture results when possible [6]. Moreover, the most recent American Urological Association (AUA) guidelines for recurrent uncomplicated UTI in women recommend ordering cultures for symptomatic patients when possible to reduce the overuse of antibiotics and development of antibiotic resistance [7].
Based on real-life practice, we have observed that for women seen early in the week, we follow current recommended guidelines to treat patients based on culture data, as patients are able to be treated urgently if they become more symptomatic prior to culture results being available. However, for those presenting later in the week, it is difficult to ensure that these women who might develop worse UTI symptoms prior to the return of their culture results over the weekend while the office is closed will be treated appropriately and avoid an urgent emergency department visit. Antibiotics chosen empirically may not prove effective against the uropathogen that ultimately grows in culture. This not only exposes the patient treated empirically to unnecessary antibiotic therapy, but also can cause side effects or full allergic reactions requiring separate management. Moreover, at times cultures return negative revealing that the antibiotic therapy was not necessary at all. This overtreatment can lead to side effects as well as the development of further bacterial resistance [4]. Therefore, the purpose of this study was to prospectively assess the benefits and risks associated with empiric prescription of antibiotic therapy for treatment of a UTI based on presentation time during the week.
Methods
Following institutional review board approval, a prospective study was performed from July 2020 to March 2022. Patients were selected from one urology clinic at a single institution. Inclusion criteria for the study included female patients 18–85 years old who presented to or notified the outpatient urology clinic of urinary symptoms suggestive of an uncomplicated UTI. Patients were defined as having recurrent UTIs (RUTI) if they had ≥ 2 symptomatic UTIs in 6 months or ≥ 3 symptomatic UTIs in a year. Patients defined as having a history of UTI had ≥ 1 lifetime UTI. Exclusion criteria included patients on antibiotics at baseline, patients on self-start antibiotic therapy, patients with neurogenic bladder conditions, indwelling urinary catheters, and/or uncontrolled diabetes (HbA1c > 8), patients from out of town for whom follow-up would not be feasible, pregnancy, patients with an allergy to nitrofurantoin (NF), individuals with altered mental status (psychosis, dementia), presentations with symptoms suggestive of pyelonephritis, and patients with swallowing disorders.
The study involved two groups of patients: a culture-based (CB) treatment group and an empiric treatment (ET) group. Patients were placed into one of the two groups based on the day of the week they contacted the clinic with their UTI symptoms: CB group on Monday and Tuesday and ET group on Wednesday, Thursday, or Friday with start on nitrofurantoin (NF) pending urine culture results in the latter cohort. NF 100 mg twice a day for 7 days was the only antibiotic used for empiric treatment in accordance with current AUA guidelines, strong safety profile, low rates of resistance, and affordability in the USA compared to other first-line therapies [7,8,9]. Patients in the CB group were given emergency room warnings and advised to utilize symptomatic therapies such as nonsteroidal anti-inflammatory medications, hydration, and phenazopyridine while awaiting culture results. Patients in the CB group were contacted after urine culture results returned (48–72 h after presentation) and treated according to culture results using same NF treatment regimen if the uropathogen isolated was susceptible to NF.
For both groups, a trained clinic representative contacted women 7 days after the final antibiotic treatment decision was made, thus at the end of their therapy. A standardized validated questionnaire, the UTI Symptom Assessment questionnaire (UTISA), was administered to inquire about UTI symptoms and any adverse effects to therapy [10]. Per the UTISA published questionnaire, symptom categories were ranked 0 (no symptoms) to 3 (severe symptoms). Any individual category score of 2 (moderate) or 3 (severe) was considered a treatment failure. If all categories were ranked either 0 (no symptoms) or 1 (mild), treatment was considered a success. Any antibiotic retreatment with NF, use of another antibiotic following completion of NF therapy, or extension of the original NF antibiotic course was also considered as treatment failure. A second phone call was made 7 days following completion of NF treatment during which the UTISA questionnaire was administered again.
Questionnaire responses and patient data were collected in a protected database and stored on a secure university server. Patient data were collected from the electronic medical record (Epic) and included demographic data (age, gender, race, body mass index), urine culture results, comorbid conditions (diabetes, renal insufficiency), hormonal status, and urologic history.
To power the study, it was assumed that the ET group would have a failure rate on NF of 25% (15% would need to change therapy upon return of the culture showing resistance to NF and 10% would show no clinical response to NF at 7 days) and the CB group would have a failure rate of 10% (no clinical response to given antibiotic at 7 days). The sample size needed to detect the difference between the two groups with 80% power at the 0.05 significance level was calculated to be 92 and 138, given a 2:3 ratio of CB to ET. To account for the loss of patients enrolled whose cultures resulted as negative, each group was increased by 5% for a total of 97 and 145 patients.
Statistics
Data were collected in Microsoft Excel (Microsoft, Redmond, WA). Descriptive statistics were provided as medians and interquartile ranges for continuous factors, and frequencies and percentages for categorical factors. Fisher’s exact test was used to compare categorical patient characteristics by treatment group. Student t-tests were used to test for differences in means for continuous patient characteristics by treatment group. As patients were treated multiple times over the course of the study, to account for the dependence of observations from the same patient, generalized estimating equations (GEE) were used to determine whether there was an association between failure at 7 days or failure at 14 days with the treatment group in which each individual patient belonged. A GEE model was also built for determining whether there was an association between the number of antibiotic class resistances and the treatment group. All analyses were two-sided and performed at the 0.05 significance level without adjustment for multiple comparisons using SAS 9.4 (SAS Institute Inc., Cary NC).
Results
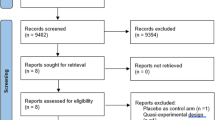
From July 2020 to March 2022, 94 women with 118 UTI events were screened. Twenty-five patients did not meet the inclusion criteria: 6 were greater than 85 years old, 9 were on baseline antibiotics at the time of screening, 3 were on self-start antibiotic regimens, 1 had neurogenic bladder, 2 had indwelling catheters, and 4 had known allergies to NF. One additional patient was excluded who did not receive NF despite being in the ET group. Given that the majority of the women were post-menopausal, 3 pre-menopausal patients were also ultimately excluded to focus the analysis on post-menopausal women. Sixty-five patients with 80 UTI events were included for final analysis, with CB treatment used for 57 UTIs and ET used for 23 UTIs. Due to the COVID-19 pandemic, we were unable to meet enrollment goals. Additionally, the reality of real-life practice led to 8 UTI events in the ET group and 24 UTI events in the CB group being given treatment that did not correspond to study methodology as to the day that they contacted the clinic.
Median age was 69 years old, with 74% of patients having had a history of RUTI and 26% a history of UTI (as defined in the methods). Patient characteristics were not significantly different between the two groups (Table 1). For the 64 UTI events with a 7-day questionnaire available, there was not a significant association between treatment group and failure, accounting for UTI events coming from the same patient (P = 0.076). The questionnaire failure rate was 44% (20/45) for the CB group and 16% (3/19) for the ET group. For the 60 UTI events with a 14-day questionnaire available, there was not a significant association between treatment group and failure (P = 0.3). The questionnaire failure rate was 31% (13/42) for the CB group and 17% (3/18) for the ET group.
In 23 cases of UTIs receiving ET, there were 5 instances where NF resistance was unable to be determined: 2 urine cultures had no growth and 3 had no culture results available for review. Out of the remaining 18 cultures from the ET group, only 2 cultures (11%) were found to have pathogens resistant to NF. There was a significant difference in the number of class resistances in the patients who received ET (3.9; 95% CI 2.7, 5.2) versus CB treatment (1.8; 95% CI 1.1, 2.6; P = 0.011). The baseline organisms present in each group were similar. In the CB group 19% (11/57) of baseline cultures were polymicrobial, 47% (27/57) grew Escherichia coli, and 14% (8/57) grew Enterococcus faecalis. In the ET group, 21% (4/19) were polymicrobial at baseline, 53% (10/19) grew Escherichia coli, and 11% (2/19) grew Enterococcus faecalis. All 7 cases of Klebsiella pneumoniae were in the CB group. Three patients reported side effects at the 7-day follow-up (all in the CB group; one with nausea and vomiting, one with stomach cramps, and one with stomachache and headache). One patient in the ET group reported side effects at her 14-day follow-up (diarrhea). All four women who reported side effects were on NF.
The same organisms from 11 UTIs were present in a follow-up culture: 9 from the CB group and 2 from ET group. The results of these cultures are shown in Table 2. There were 5 additional follow-up cultures that had new organisms compared to baseline: 5 in the CB group and 1 in the ET group.
Discussion
This study examined the real-world use of empiric and culture-based antibiotic therapy in an ambulatory urology tertiary care urology practice. Due to COVID-19, the study was terminated before all expected goals were met. Nonetheless, we found no significant difference in treatment outcomes between empiric and culture-based treatment of UTIs.
We chose to administer the UTISA questionnaire at 7 and 14 days following start of treatment given the questionnaire’s ability to detect small changes in symptoms following completion of therapy for a UTI [10]. While not significantly different, questionnaire failure rate at 7 days was more than double (44% vs. 16%) in the CB group compared to the ET group. At 14 days, there was only a 14% difference in the failure rate between the two groups (31% CB vs. 17% ET). These observations suggest that treating patients empirically may lead to a faster improvement in UTI-related symptoms. This is presumably due to a lesser amount of planktonic bacterial load to eradicate early on, as opposed to a larger amount when the infection has had more time to develop. In addition, no patients in the study reported hospitalization due to urosepsis or pyelonephritis during the study duration. This is consistent with recently reported findings that only 0.3% of patients presenting in an outpatient setting for acute cystitis progress to sepsis within 7 days [11].
Culture-based therapy continues to be the gold standard for UTI therapy given the risk of over or inappropriate treatment with empiric antibiotics [12]. We found that of 23 patients treated empirically, 18 (78%) had positive culture results with 2/18 (11%) of these cultures revealing pathogens with NF resistance requiring a change in antibiotic therapy. In a recent retrospective review, Dokter et. al reported that of women treated empirically for suspected UTIs, 43% of cultures were positive with 26% of empirically treated patients requiring a change in antibiotics due to bacterial resistance on culture [13]. This study is comparable to our findings as it occurred in an outpatient clinic run by Female Pelvic Medicine and Reconstructive Surgery (FPMRS)-trained urologists. The higher culture positivity rates in our study may be due to the high number of women with a history of RUTIs.
In this study, we chose to treat all patients in the ET group with NF given its strong safety profile, low rates of resistance, and affordability compared to other first-line therapies [8, 9]. In a randomized trial comparing NF to fosfomycin, Huttner et al. found that NF led to a resolution of UTI symptoms in 70% of patients at 28 days with side effects reported in 4% of patients [14]. While our rates of NF resistance were higher than previously reported, bacteria isolated from urine cultures of the patients in the ET group did have significantly more class resistances than bacteria cultured from the urine of patients in the CB group. The higher rates of resistance in the ET group may indicate that women who received ET had greater exposure to antibiotics in the past.
Antibiotic resistance is increasing with the most recent data from the U.S. Center for Disease Control and Prevention reporting over 2 million people in the USA diagnosed with an antibiotic-resistant infection annually. Approximately 23,000 of these antibiotic-resistant cases have resulted in death [15,16,17]. UTIs with highly resistant bacteria are estimated to account for $20 billion in direct healthcare costs per year [18]. In practice many providers utilize ET, current guidelines from the IDSA and AUA do not provide clear guidance on the appropriate use of ET [7, 12].
With the increased use of telehealth resulting from the COVID-19 pandemic, ET is likely to continue to be common practice for the treatment of uncomplicated cystitis. Larger-scale studies are needed to determine when ET is indicated. This study may help in informing future guidelines that provide specific recommendations on the use of ET for uncomplicated cystitis. The limitations of this study include the inclusion of mainly Caucasian menopausal women at a tertiary center, FPMRS-based, practice. Furthermore, participation in this study was stopped before reaching enrollment goals due to low numbers related to the COVID-19 pandemic. Additionally, due to continuous flux of staff during this period, proper education of clinic staff about study procedures for selecting ET or CB treatment based on the day of the week proved untenable.
Conclusion
In a limited prospective cohort study, outcomes following the use of NF for the empiric treatment of uncomplicated cystitis were not significantly different when compared to patients receiving culture-based treatment. UTI symptoms were relieved faster in the ET group. These findings do not deter from the empiric use of NF for the treatment of uncomplicated cystitis in this selected group.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Schappert SM, Rechtsteiner EA (2011) Ambulatory medical care utilization estimates for 2007. Vital Health Stat 169:1–38
Kodner CM, Thomas Gupton EK (2010) Recurrent urinary tract infections in women: diagnosis and management. Am Fam Physician 82(6):638–643
O’Brien K, Hillier S, Simpson S, Hood K, Butler C (2007) An observational study of empirical antibiotics for adult women with uncomplicated UTI in general practice. J Antimicrob Chemother 59(6):1200–1203. https://doi.org/10.1093/jac/dkm108
Abbo LM, Hooton TM (2014) Antimicrobial stewardship and urinary tract infections. Antibiotics 3(2):174–192. https://doi.org/10.3390/antibiotics3020174
Miller JM, Binnicker MJ, Campbell S et al (2018) A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 67(6):e1–e94
Dason S, Dason JT, Kapoor A (2011) Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J 5(5):316
Anger J, Lee U, Ackerman AL et al (2019) Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol 202(2):282–289. https://doi.org/10.1097/ju.0000000000000296
Sanchez GV, Baird AM, Karlowsky JA, Master RN, Bordon JM (2014) Nitrofurantoin retains antimicrobial activity against multidrug-resistant urinary Escherichia coli from US outpatients. J Antimicrob Chemother 69(12):3259–3262. https://doi.org/10.1093/jac/dku282
McKinnell JA, Stollenwerk NS, Jung CW, Miller LG (2011) Nitrofurantoin compares favorably to recommended agents as empirical treatment of uncomplicated urinary tract infections in a decision and cost analysis. Mayo Clin Proc 86(6):480–488. https://doi.org/10.4065/mcp.2010.0800
Clayson D, Wild D, Doll H, Keating K, Gondek K (2005) Validation of a patient-administered questionnaire to measure the severity and bothersomeness of lower urinary tract symptoms in uncomplicated urinary tract infection (UTI): the UTI Symptom Assessment Questionnaire. BJU Int 96(3):350–359. https://doi.org/10.1111/j.1464-410X.2005.05630.x
Kaefer D, Chiang J, Ackerman AL (2022) PD32-05 rate of progression to sepsis following presentation for acute cystitis. J Urol 207(Supplement 5):e548. https://doi.org/10.1097/JU.0000000000002583.05
Gupta K, Hooton TM, Naber KG et al (2011) International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52(5):e103–e120. https://doi.org/10.1093/cid/ciq257
Dokter J, Tennyson LE, Nguyen L, Han E, Sirls LT (2020) The clinical rate of antibiotic change following empiric treatment for suspected urinary tract infections. Int Urol Nephrol 52(3):431–436. https://doi.org/10.1007/s11255-019-02327-7
Huttner A, Kowalczyk A, Turjeman A et al (2018) Effect of 5-day nitrofurantoin vs single-dose fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women: a randomized clinical trial. JAMA 319(17):1781–1789. https://doi.org/10.1001/jama.2018.3627
Medina M, Castillo-Pino E (2019) An introduction to the epidemiology and burden of urinary tract infections. Therapuetic Advance ini Urology 11:1756287219832172. https://doi.org/10.1177/1756287219832172
Centers for disease control and prevention: antibiotic resistance threats in the United States. Prevention CfDCa; 2019. https://www.cdc.gov/drugresistance/pdf/threatsreport/2019-ar-threats-report-508.pdf. Accessed Sept 2022
Kadri SS (2020) Key takeaways from the U.S. CDC’s 2019 antibiotic resistance threats report for frontline providers. Crit Care Med 48(7):939–945. https://doi.org/10.1097/ccm.0000000000004371
Dadgostar P (2019) Antimicrobial resistance: implications and costs. Infect Drug Resist 12:3903–3910. https://doi.org/10.2147/idr.S234610
Funding
The authors did not receive support from any organization for the submitted work (IRB STU-2020-0033).
Author information
Authors and Affiliations
Contributions
SBK: data collection, data analysis, manuscript writing. EMF: Project development, data collection. BCP: project development, manuscript writing. ALC: project development, data analysis, manuscript writing. PEZ: project development, data analysis, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kusin, S.B., Fan, E.M., Prokesch, B.C. et al. Empiric versus culture-based antibiotic therapy for UTIs in menopausal women. World J Urol 41, 791–796 (2023). https://doi.org/10.1007/s00345-023-04303-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04303-4