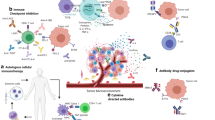
Abstract
Purpose
Prostate cancer (PCa) is the most common malignancy in men and the cause for the second most common cancer-related death in the western world. Despite ongoing development of novel approaches such as second generation androgen receptor targeted therapies, metastatic disease is still fatal. In PCa, immunotherapy (IT) has not reached a therapeutic breakthrough as compared to several other solid tumors yet. We aimed at highlighting the underlying cellular mechanisms crucial for IT in PCa and giving an update of the most essential past and ongoing clinical trials in the field.
Methods
We searched for relevant publications on molecular and cellular mechanisms involved in the PCa tumor microenvironment and response to IT as well as completed and ongoing IT studies and screened appropriate abstracts of international congresses.
Results
Tumor progression and patient outcomes depend on complex cellular and molecular interactions of the tumor with the host immune system, driven rather dormant in case of PCa. Sipuleucel-T and pembrolizumab are the only registered immune-oncology drugs to treat this malignancy. A plethora of studies assess combination of immunotherapy with other agents or treatment modalities like radiation therapy which might increase its antineoplastic activity. No robust and clinically relevant prognostic or predictive biomarkers have been established yet.
Conclusion
Despite immunosuppressive functional status of PCa microenvironment, current evidence, based on cellular and molecular conditions, encourages further research in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite promising results of immunotherapy (IT) in genitourinary malignancies such as urothelial and kidney cancer, IT has not turned out to be a meaningful player in the treatment armamentarium of advanced prostate cancer (PCa) yet. The only registered agent in the field, sipuleucel-T, an immunostimulant based on dendritic cells, has shown a benefit in overall survival (OS) of almost 4 months compared to placebo in metastasized castration-resistant PCa (mCRPC) [1]. However, a serious drawback was that a viral vector-based IT approach reported in the PROSTVAC-trial could not show any positive effect on OS in the most recent update [2]. In addition, development of novel treatment strategies such as androgen receptor targeted therapies (ART) has shifted the clinical focus somewhat away from IT in advanced PCa.
Bearing in mind the robust advances made with the development of programmed cell death ligand-1 (PD-L1) and programmed cell death-1 receptor (PD-1) inhibitors in a number of solid malignancies, immune oncology remains an essential part of the current research activities in PCa, despite the fact that PCa is considered a non-immunoreactive and a “cold” tumor with an immunosuppressive tumor microenvironment (TME) and low infiltration burden of T cells. For instance, pembrolizumab is presently under investigation in combination with several other standard of care regimens such as secondary ARTs (ClinicalTrials.gov: NCT02787005) or poly(ADP-ribose) polymerase (PARP) inhibitors (ClinicalTrials.gov: NCT03834519).
Within the complexity of the immune system and involvement of a multitude of immune cells, key enzymes and receptors, the immunotherapeutic approach remains a highly appealing strategy to optimize treatment for patients with PCa. In this review, we highlight the current underlying mechanisms of immunotherapy in cancer, with a focus on PCa. Furthermore, we provide an overview of the relevant clinical trials that have the potential to reshape the landscape of PCa treatment in the near future.
Materials and methods
Between June 26 and July 11, 2020, we searched Medline, Embase and other databases as well as the Google web search engine for peer-reviewed articles and published abstracts from international congresses in English using the terms “prostate cancer” and “immune therapy”, “immunotherapy”, “immune-oncology drug”, “vaccine” as well as “checkpoint inhibitor”. Furthermore, we searched ClinicalTrials.gov for clinical trials evaluating immunotherapy in PCa that have been recently completed, are ongoing or are actively recruiting participants.
Molecular aspects of the immune contexture in prostate cancer
Tumor microenvironment and infiltrating immune cells
Cancer formation and progression strongly depend on the TME in which it develops [3]. Besides tumor cells, solid malignancies are composed of a number of other cells including fibroblasts, endothelial cells, innate and adaptive immune cells, extracellular matrix as well as extracellular soluble molecules like cytokines, chemokines, growth factors and metabolic products [4]. Importantly, PCa is known to be a "cold" tumor with a low T-cell infiltration. Thereby, effective immune response counteracting tumor progression presupposes activation of cancer-combatting host immune cells, their enrichment at the tumor sites and overcoming the dormant impact of tumor-associated immunosuppressive cells on the TME, mediated by secreted and cellular factors [5]. In an attempt to establish a tumor-agnostic, prognostic and predictive biomarker based on the immune contexture, “Immunoscore” has been developed in the area of colorectal cancer [6,7,8]. It relies on the quantification of lymphocyte populations, in particular CD3+ and CD8+ T cells, counted at the tumor center and at the invasive margin. Thereby, increasing score correlates with a longer patient survival [3] and was also supposed to predict response to immune checkpoints inhibitors targeting PD-1/PD-L1 or CTLA4 [7, 9]. Another approach to predict response to immunotherapy is the “tumor inflammation signature” (TIS). This 18-gene signature measures the level of T-cell inflammation as an immune-phenotyping tool across different histologically defined tumor types [10]. Analysis of 9,083 samples of 32 cancers, including PCa, demonstrated that tumors with a known clinical sensitivity to PD-1 blockade had a higher TIS average [10]. On the whole, utilization of these tools allows to classify solid malignancies into T-cell inflamed/“hot” and non-T-cell inflamed/“cold tumors” [5, 10].
Immune contexture determined by the density, composition, functional state and organization of the tumor infiltrating immune cells can yield information relevant for prognosis and treatment response [11]. For example, a positive association of the level of stromal tumor infiltrating lymphocytes (TILs) with adjuvant chemotherapeutic response in ovarian high-grade serous carcinoma has been observed [12]. Moreover, Shibutani and collaborators presented evidence for a significantly higher chemotherapeutic response rate and better progression-free survival in patients with stage IV colorectal cancer revealing a high number of TILs [13]. Indeed, the presence of cytotoxic and helper T cells within the tumor center or invasive margin has been linked to favorable outcomes in a plethora of malignancies [5, 11]. Idos et al. reported favorable outcomes in colon cancer when high levels of TILs, presence of CD3+, CD8+ and FOXP3 + cells at the tumor center and CD3+ at the invasive margin of the tumor, were observed [14]. In line with this, an association of a high tumor CD8+ T-cell density with a longer overall survival in non-small cell lung cancer has been demonstrated [15]. However, opposing results have also been reported. Kim and coauthors have recently demonstrated that a high CD8+ expression predicted independently for a shorter disease-free survival of breast cancer patients [16]. Triozzi et al. postulated a crucial role of CD8+ regulatory T cells in fostering uveal melanoma disease progression, while TIL infiltration correlated with a worse prognosis in this disease [17]. Thus, impact of CD8+ TILs might be entity specific or depend on additional factors.
Tumor microenvironment and prostate cancer
Observations regarding prognostic value of CD8+ TIL infiltration in PCa are contradictory. Ness and coauthors demonstrated that a high density of CD8+ TILs in the primary PCa specimens is an independent negative prognostic factor for biochemical failure-free survival [18]. In concert with this, a high density of CD8+ TILs and PD-L1 expression by tumor cells has been associated with a higher risk of clinical progression in men with node-positive PCa [19]. On the contrary, Yang et al. recently demonstrated that a high number of CD8+ TILs at prostatectomy is independently associated with improved survival in this majority of a high-risk PCa population [20]. In line, Vicier and collaborators reported that a high PD-L1 and low CD8+ TIL density are markers for poor prognosis and biochemical and metastatic relapse in PCa [21]. Thus, aside CD8+, additional parameters seem to be important for prognosis. Indeed, the ability of CD8+ effector T cells to promote tumor regression is largely dependent on their cytokine secretion profile and their ability to self-renew [22]. Emerging evidence demonstrates that the TME can provoke emergence of dysfunctional CD8+ T cells with a limited cytotoxic function [22]. Furthermore, senescent, regulatory, and dysfunctional stem-cell like memory CD8+ T-cell phenotypes, which do not exert antitumor activity, might coexist. Thus, a deeper profiling of the functional status and subsets of infiltrating immune cells, beyond a general surface labelling, and their spatial distribution, is required to utilize TILs as a predictive or prognostic marker.
The same holds true for different types of tumor-associated macrophages. PCa cells and cancer-associated fibroblasts stimulate monocyte recruitment toward tumor cells and their trans-differentiation into anti-inflammatory and tumor-promoting M2 macrophage phenotype [23]. Comito and collaborators yielded evidence for a more favorable biochemical recurrence-free survival of PCa patients with M1 macrophage (pro-inflammatory and tumor-suppressive) prevalence in prostatectomy specimens, compared to those with M2 macrophage prevalence [23]. TGF-beta, typically secreted by M2 macrophages, promotes various tumorigenic processes like recruitment of mesenchymal stem cells, their activation into cancer-associated fibroblasts, or PI3K-AKT signaling, fostering migration of PCa cells [24, 25]. As TGF-beta is important for immune exclusion, it might represent one essential aspect of a reduced infiltration of TILs and immunosuppressive TME in PCa [26].
Tumor mutational burden and PD1/PD-L1 signaling
PCa is characterized by a low tumor mutational burden (TMB), thus revealing a poor collection of neoepitopes crucial for immune cell attraction to the tumor sites, epitope–MHC interactions and activation of TILs by antigen-presenting cells [5, 27]. PCa has distinctly fewer mutations (0.7 per Mb) than breast (1.2 per Mb), bladder (7.1 per Mb) and colorectal cancer (3.1 per Mb), or melanoma (12.1 per Mb) [28]. Even in castration-sensitive or -resistant disease, TMB is only as high as 2.08 and 4.02 per Mb, respectively [29]. Due to a low TMB and T-cell-mediated inflammation, the probability that PCa responses to anti-PD1/PD-L1 treatment is weak [30].
It has been speculated that PCa in general is associated with a low expression of PD-L1 due to few effector T cells secreting proinflammatory cytokines [31]. Analysis of primary PCa specimens by Xian and coworkers revealed only 17.9% PD-L1 positivity [32]. In males with advanced tumor stage, lymph node metastasis, and high Gleason score more PD-L1+ tumors were found. Moreover, Haffner et al. demonstrated that PD-L1 positivity counts 7.7% in primary PCa, while it increased to 31.6% in mCRPC [33]. Bishop and coauthors showed that patients progressing on enzalutamide had a higher number of PD-L1/2+ dendritic cells in blood compared to those naïve or responding to treatment, and a high frequency of PD-1 + T cells [34]. In contrast to this, tumor specimens from men with intermediate- to high-risk PCa pretreated with abiraterone acetate prior to radical prostatectomy yielded less CD8+ T cells and a trend for decreased PD-L1 positivity (7% vs. 21%; p = 0.062), compared to untreated PCas [35]. Thus, there are considerable differences in the expression of PD-1/PD-L1 in PCa depending on tumor stage, previous treatment, and methodological issues. Further research is warranted to clarify and generalize prognostic and predictive value of these immune checkpoints.
HLA alteration
Attenuating HLA class I proteins, which are commonly abundant on nucleated cells and present intracellular peptides to T lymphocytes, is an established escape mechanism of tumor cells from cytotoxic T cells in different cancers, and associated with unfavorable clinical course and resistance to immunotherapy [36]. A complete loss and, in case of individual allelic expression, a minimal estimated downregulation of HLA class I in 34 and 85% of primary PCas and in 80 and 100% of lymph node metastases, respectively, has been shown [37]. Moreover, downregulation of several components of HLA class I antigen processing machinery and association with a higher Gleason score and an early disease recurrence has been reported in PCa [38]. Interestingly, treating PCa cells with IFN-γ, crucial for efficient antitumor immune response and normally secreted by cytotoxic T lymphocytes, resulted in upregulation of HLA class I [39, 40]. Similarly, reversion of defects in HLA class I expression and survival improvement by IFN-γ treatment in a mouse model of PCa was demonstrated [41]. Whether therapeutically induced augmentation of the expression of HLA class 1 proteins in humans may shift the functionally immunosuppressive TME with a low TIL density towards immunoactive setting remains questionable [5]. Taken together, tumor progression and patient outcomes depend on complex cellular and molecular interactions of the tumor with the host immune system [42].
Clinical utilization of immunotherapy in prostate cancer and future directions
Currently, IT is used for patients with specific mutations but also for general PCa population, alone or in combination. A selection of phase 2 and 3 studies is shown in Table 1.
Vaccines
Active cellular ITs, named therapeutic cancer vaccines, have been tested as PCa therapy. Sipuleucel-T is a personalized therapy, made from patient’s peripheral blood mononuclear cells incubated with a fusion protein consisting of a common prostate cancer antigen (prostatic acid phosphatase) linked to an adjuvant (granulocyte–macrophage colony-stimulating factor). Infused into the patient, it induces CD4+ and CD8+ immune cells against the tumor antigen. The main phase 3 trial randomly assigned 512 patients with mCRPC and an expected survival of at least 6 months to receive sipuleucel-T or placebo [1]. With 34.1 months median follow-up, OS was 25.8 months in the sipuleucel-T group vs 21.7 in placebo group. However, there was no difference regarding the time to objective disease progression (14.6 in sipuleucel-T vs 14.4 months in placebo groups, respectively). These results were consistent with two previous phase 3 studies [43, 44]. To date, sipuleucel-T remains the only approved vaccine therapy for PCa. Importantly, this medicine is withdrawn from the use in Europe. All-in-all, its complex administration, high price, supply problems due to a limited manufacturing capacity and uncertainty about the reimbursement status hampered its prescription resulting in bankruptcy of its owner Dendreon [45].
PROSTVAC utilizes recombinant poxviruses that express PSA with immune-enhancing costimulatory molecule to stimulate immune response. A phase 2 trial showed a median survival improvement of 8.2 months (p = 0.0061) although this was not the primary endpoint [46]. Subsequently, a phase 3 trial [2] did not support the initially positive signal of the phase 2 study with no effect on OS and progression free survival (PFS) in mCRPC patients.
GVAX consists of two metastatic prostate cancer cell lines transfected with a human GM-CSF gene. GVAX injection breaks immune tolerance to antigens expressed by prostate cancer and induces antitumor immune responses. Two phase 3 trials have been initiated (VITAL1 and 2) to compare GVAX to docetaxel plus prednisone in asymptomatic and symptomatic metastatic prostate cancer patients but were both stopped for futility and an increase in mortality.
Checkpoint inhibitors
Ipilumumab is a CTLA-4 inhibitor. A phase 2 trial enrolled 30 patients with mCRPC [47]. With a median follow-up of 45.5 months, median radiographic PFS and OS were 3 months and 24.3 months, respectively. Overall, 28% of patients receiving treatment experienced grade 3 adverse effects (AE) without any grade 4–5 cases. A favorable cohort has been identified expressing a higher density of cytotoxic and memory T cells in the tumor and an increased expression of IFN-γ signaling suggesting the potential role of these biological markers as theranostic factors.
Ipilimumab has also been evaluated in a phase 3 trial (CA184-095) randomizing 602 chemonaïve patients with asymptomatic or minimally symptomatic mCRPC without visceral metastasis to receive ipilimumab or placebo in a 2:1 ratio [48]. No significant difference in OS was observed between arms (27.8 vs. 29.7 months, respectively). However, ipilimumab was associated with a longer median PFS (5.6 vs 3.8 months). AEs grade 4–5 were observed in 27% patients in ipilimumab group versus 2% in the placebo arm. This large trial did not conclusively demonstrate an ipilimumab-driven benefit for OS.
Loss of function in mismatch repair (MMR) genes has been associated with favorable responses to PD-1 blockade immunotherapy in different cancer types including PCa [49]. In a case series, anti PD1/PD-L1 was used in 11 men with mCRPC. Overall, five patients had durable clinical benefit, five had no benefit, and one had stable disease for approximately 6 months [50].
Pembrolizumab has been assessed in the phase 2 KEYNOTE-199 study among 258 patients with mCRPC previously treated with docetaxel and at least one hormonal therapy [51]. Cohort 1 and 2 included any measurable disease with PD-L1 positive and PD-L1 negative patients, cohort 3 included patients with bone predominant disease, regardless of PD-L1 expression. Median follow-up was 16.8 months. Objective response rate (ORR) was 5% in cohort 1 and 3% in cohort 2 with a median OS of 9.5 and 7.9 months, respectively. Median OS in cohort 3 was 14.1 months. Pembrolizumab monotherapy showed encouraging results for antitumor activity and disease control with an acceptable safety profile, pushing for additional investigations. Based on these results, Food and Drug Administration (FDA) approved pembrolizumab for the treatment of mCRPC with MMR deficiency or high microsatellite instability.
Avelumab is a PD-L1 inhibitor. The phase 1 trial reported in mCRPC patients included 18 patients, while seven had stable disease after 24 weeks of treatment [52]. Avelumab was safe and tolerable with 15 patients experiencing grade 1–2 AEs and only one grade 3 AE. Immune analysis and other studies are awaited to determine which patients would benefit most from this treatment.
Combinations
Immunotherapy combination
Nivolumab, a PD-1 inhibitor, has been combined with ipilimumab in different cancers. CheckMate 650 is a phase 2 study of nivolumab plus ipilimumab for the treatment of mCRPC [53]. This study involved two cohorts. The first cohort included asymptomatic or minimally symptomatic patients who progressed after second generation hormone therapy (no prior chemotherapy) and the second cohort included patients who progressed after taxane-based therapy. Objective response rate (ORR) was 26% in cohort 1 and 10% in cohort 2. PSA decline > 50% was observed in 21% of cases in cohort 1 and 13% of cases in cohort 2. A PD-L1 ≥ 1% expression, the presence of DNA damage repair, a homologous recombination deficiency, or an above-median tumor mutational burden were associated with higher ORR. Grade 3–4 AEs occurred in 39 and 51% of patients in cohorts 1 and 2, respectively, with one death reported in each cohort.
Another phase 2 trial used nivolumab plus ipilimumab for patients with ARV7+ mCRPC [54]. Overall, 15 patients were enrolled. With a median follow-up of 8.4 months, 7% had PSA response rate and ORR was 25%. OS was 9.5 months, whereas patients with DNA repair deficient tumors had a more favorable biochemical and radiographic PFS. This combination revealed acceptable safety and encouraging efficacy, particularly in men with DNA repair deficient tumors.
CTLA-4 blockade could enhance antitumor immunity when combined with cancer vaccines. A phase 1–2 trial included 28 patients with asymptomatic chemonaive mCRPC treated with a combination of GVAX and ipilimumab [55]. OS was 29 months and 32.1% patients had a PSA response.
Pre-treatment high levels of CD4+, CTLA-4+, CD4+PD-1+, non-naive CD8+ and low frequencies of CD4+ or regulatory T cells were associated with a significantly prolonged OS. OS was extended when treatment induced > 25% increase in lymphocyte counts, > 30% increase in non-naive (memory) CD4+ T cells, CD4+ and CD8+ T-cell activation.
ART and immunotherapy
ART such as enzalutamide might improve IFN-γ levels and sensitize tumor cells to immune-mediated cell-killing. Immunotherapy associated with enzalutamide seems to be an interesting combination for patients with PCa.
Atezolizumab is a humanized monoclonal antibody which inhibits the interaction between PD-L1 and its receptor. The combination of atezolizumab and enzalutamide has been examined in the phase III randomized trial IMbassador250 evaluating the combined treatment vs enzalutamide alone in 759 mCRPC or locally advanced CRPC patients who had progressed on abiraterone and docetaxel, or who were not candidates for a taxane regimen. Overall survival was not different between arms discouraging the use of the combination [56].
In the above-mentioned multicohort phase 2 study KEYNOTE-199, the cohorts 4 (measurable disease) and 5 (bone predominant disease) received a combination of pembrolizumab and enzalutamide [57]. After a median follow-up of 13.7 months, most patients had disease progression. Disease control rate was 51%. ORR for patients with measurable disease was with 12% relatively low. However, duration of response was almost 6 months in 60% of responders.
Immunotherapy and PARP inhibitor
Durvalumab is a human IgG1-K monoclonal antibody that targets PD-L1. Olaparib is a PARP inhibitor approved for patients with mCRPC carrying homologous recombination repair gene alteration. Preclinical data have suggested a synergistic effect between PARP and checkpoint inhibitors. A phase II, open-label trial has assessed this combination in multiple cohorts of heavily pretreated mCRPC patients [58]. Seventeen patients were enrolled and received durvalumab plus olaparib. Median rPFS was 16.1 months. The 1-year PFS rate was 83.3% for patients with alteration in DNA damage response gene vs. 36.4% for those without mutations (p = 0.031). Most common treatment-related grade 3 or 4 AEs were anemia, lymphopenia, infection, and nausea.
Immunotherapy and local treatments
Radiation treatment may have a systemic role by activating the immune system, stimulating immune priming (abscopal effect), and improving response to immunotherapy.
A phase 1–2 study assessing ipilimumab ± radiotherapy in mCRPC disease showed a 29% rate of non-progressive disease with the treatment combination after a median follow-up of 15.7 months [59]. In this trial, metastasis-directed radiotherapy was given at a single dose of 8 Gy per target bone lesion (for up to three lesions per patient) at 24–48 h before the first ipilimumab dose.
A phase 3 study included patients with post-docetaxel mCRPC and bone metastasis and randomized patients to receive bone-directed radiotherapy with ipilimumab or placebo [60]. All patients received a single dose of radiotherapy of 8 Gy for at least one, and up to five, bone lesions. Radiotherapy was done within the 2 days before initiation of the study drug regimen, and palliative radiotherapy was allowed for any bone lesion while on study. The median follow-up was 9.9 months in ipilimumab group and 9.3 months in placebo group. Median overall survival was 11.2 and 10.0 months [HR 0.85], respectively, without significant difference (p = 0.053). Median PFS was 4.0 months for ipilimumab group vs. 3.1 for placebo group [HR 0.70, p < 0.001]. Post hoc analysis suggested that ipilimumab might provide the most benefit in patients with favorable prognostic features, specifically in patients without visceral metastasis. The rates of grade 3–4 AEs were 59% for ipilimumab group vs 41% for placebo group.
Cryotherapy might also help to increase the immunogenicity of PCa. A pilot trial has evaluated the role of prostate cryotherapy, short term androgen deprivation and pembrolizumab in oligometastatic PCa [61]. After 1 year, 42% of patients had a PSA level below 0.6 ng/mL for a median PSA-PFS of 14 months. Median systemic therapy-free survival was 17.5 months. No grade 3 or more AEs was observed.
Ongoing trials
Multiple clinical trials are ongoing assessing immunotherapy drugs and vaccines for the management of prostate cancer (Table 2). IT is currently evaluated alone or in combination with life-prolonging or investigational drugs, from localized to late-stage mCRPC.
However, to date, even if many strategies are promising, sipuleucel-T and pembrolizumab remain the only IT strategies approved by the FDA without widespread use in routine practice. Management of metastatic PCa is a rapidly evolving field. Recently, several drugs have been proven to be life-prolonging, including new hormone therapies, theranostic radioligands and PARP inhibitors. The choice of the ideal treatment for an individual patient will be probably guided in a close future by the validation of predictive factors of response/resistance to avoid ineffective therapy and to prolong tumor response. By considering that perspective, the use of immunotherapy could be driven by the quantification of lymphocytes populations with the example of the immunoscore developed in colorectal cancer [6,7,8]. The level of expression of immunoregulators in tumor tissue could be also relevant to anticipate response to immune checkpoints inhibitors targeting PD-1/PD-L1 or CTLA4. However, to date, no biological or immunological signature has been validated in PCa to guide this immunotherapy choice.
Conclusions
The relevance of IT strategies in the treatment course of PCa is still ambiguous. Progress in translational research and results from ongoing large 2 and 3 trials are urgently awaited to draw clinically applicable conclusions.
Change history
13 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00345-021-03849-5
References
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422
Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J, Saad F, Shore ND, Chen L, Heery CR, Gerritsen WR, Priou F, Langkilde NC, Novikov A, Kantoff PW (2019) Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol 37:1051–1061
Angell HK, Bruni D, Barrett JC, Herbst R, Galon J (2020) The Immunoscore: colon cancer and beyond. Clin Cancer Res 26:332–339
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24:541–550
Vitkin N, Nersesian S, Siemens DR, Koti M (2019) The tumor immune contexture of prostate cancer. Front Immunol 10:603
Galon J, Fridman WH, Pages F (2007) The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res 67:1883–1886
Angell H, Galon J (2013) From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 25:261–267
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12:298–306
Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA (2012) The immune score as a new possible approach for the classification of cancer. J Transl Med 10:1
Danaher P, Warren S, Lu R, Samayoa J, Sullivan A, Pekker I, Wallden B, Marincola FM, Cesano A (2018) Pan-cancer adaptive immune resistance as defined by the Tumor Inflammation Signature (TIS): results from The Cancer Genome Atlas (TCGA). J Immunother Cancer 6:63
Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G (2017) The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14:717–734
Choi KU, Kim A, Kim JY, Kim KH, Hwang C, Lee SJ, Park WY, Jung S, Choi HJ, Kim K (2020) Differences in immune-related gene expressions and tumor-infiltrating lymphocytes according to chemotherapeutic response in ovarian high-grade serous carcinoma. J Ovarian Res 13:65
Shibutani M, Maeda K, Nagahara H, Fukuoka T, Iseki Y, Matsutani S, Kashiwagi S, Tanaka H, Hirakawa K, Ohira M (2018) Tumor-infiltrating lymphocytes predict the chemotherapeutic outcomes in patients with stage iv colorectal cancer. Vivo 32:151–158
Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, Qu C (2020) The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: a systematic review and meta-analysis. Sci Rep 10:3360
Hao J, Wang H, Song L, Li S, Che N, Zhang S, Zhang H, Wang J (2020) Infiltration of CD8(+) FOXP3(+) T cells, CD8(+) T cells, and FOXP3(+) T cells in non-small cell lung cancer microenvironment. Int J Clin Exp Pathol 13:880–888
Kim HM, Koo JS (2020) Clinicopathologic characteristics of breast cancer according to the infiltrating immune cell subtypes. Int J Mol Sci. https://doi.org/10.3390/ijms21124438
Triozzi PL, Schoenfield L, Plesec T, Saunthararajah Y, Tubbs RR, Singh AD (2019) Molecular profiling of primary uveal melanomas with tumor-infiltrating lymphocytes. Oncoimmunology 8:e947169
Ness N, Andersen S, Valkov A, Nordby Y, Donnem T, Al-Saad S, Busund LT, Bremnes RM, Richardsen E (2014) Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 74:1452–1461
Petitprez F, Fossati N, Vano Y, Freschi M, Becht E, Luciano R, Calderaro J, Guedet T, Lacroix L, Rancoita PMV, Montorsi F, Fridman WH, Sautes-Fridman C, Briganti A, Doglioni C, Bellone M (2019) PD-L1 expression and CD8(+) T-cell infiltrate are associated with clinical progression in patients with node-positive prostate cancer. Eur Urol Focus 5:192–196
Yang Y, Attwood K, Versaggi C, Omilian A, Bshara W, Xu B, Kauffman E, Mohler J, Guru K, Li Q, George S, Basse P, Morrison C, Papanicolau-Sengos A, Kalinski P, Chatta GS (2018) Association of high CD8+ tumor infiltrating lymphocytes at prostatectomy with improved survival of prostate cancer patients. J Clin Oncol 36:5068–5068
Vicier C, Werner L, Huang Y, Hamid A, Evan C, Loda M, Sweeney C (2019) Immune infiltrate with CD8 low or PDL1 high associated with metastatic prostate cancer after radical prostatectomy (RP). J Clin Oncol 37:86–86
Apetoh L, Smyth MJ, Drake CG, Abastado JP, Apte RN, Ayyoub M, Blay JY, Bonneville M, Butterfield LH, Caignard A, Castelli C, Cavallo F, Celis E, Chen L, Colombo MP, Comin-Anduix B, Coukos G, Dhodapkar MV, Dranoff G, Frazer IH, Fridman WH, Gabrilovich DI, Gilboa E, Gnjatic S, Jager D, Kalinski P, Kaufman HL, Kiessling R, Kirkwood J, Knuth A, Liblau R, Lotze MT, Lugli E, Marincola F, Melero I, Melief CJ, Mempel TR, Mittendorf EA, Odun K, Overwijk WW, Palucka AK, Parmiani G, Ribas A, Romero P, Schreiber RD, Schuler G, Srivastava PK, Tartour E, Valmori D, van der Burg SH, van der Bruggen P, van den Eynde BJ, Wang E, Zou W, Whiteside TL, Speiser DE, Pardoll DM, Restifo NP, Anderson AC (2015) Consensus nomenclature for CD8(+) T cell phenotypes in cancer. Oncoimmunology 4:e998538
Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P (2014) Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 33:2423–2431
Barcellos-de-Souza P, Comito G, Pons-Segura C, Taddei ML, Gori V, Becherucci V, Bambi F, Margheri F, Laurenzana A, Del Rosso M, Chiarugi P (2016) Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-beta1. Stem Cells 34:2536–2547
Hamidi A, Song J, Thakur N, Itoh S, Marcusson A, Bergh A, Heldin CH, Landstrom M (2017) TGF-beta promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85alpha. Sci Signal. https://doi.org/10.1126/scisignal.aal4186
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Senbabaoglu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Hoglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T (2018) TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554:544–548
De Velasco MA, Uemura H (2018) Prostate cancer immunotherapy: where are we and where are we going? Curr Opin Urol 28:15–24
Frank S, Nelson P, Vasioukhin V (2018) Recent advances in prostate cancer research: large-scale genomic analyses reveal novel driver mutations and DNA repair defects. F1000Res. https://doi.org/10.12688/f1000research.14499.1
Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, Danila D, Rathkopf D, Morris M, Slovin S, McLaughlin B, Curtis K, Hyman DM, Durack JC, Solomon SB, Arcila ME, Zehir A, Syed A, Gao J, Chakravarty D, Vargas HA, Robson ME, Joseph V, Offit K, Donoghue MTA, Abeshouse AA, Kundra R, Heins ZJ, Penson AV, Harris C, Taylor BS, Ladanyi M, Mandelker D, Zhang L, Reuter VE, Kantoff PW, Solit DB, Berger MF, Sawyers CL, Schultz N, Scher HI (2017) Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis Oncol. https://doi.org/10.1200/PO.17.00029
Maleki Vareki S (2018) High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer 6:157
Cha HR, Lee JH, Ponnazhagan S (2020) Revisiting immunotherapy: a focus on prostate cancer. Cancer Res 80:1615–1623
Xian P, Ge D, Wu VJ, Patel A, Tang WW, Wu X, Zhang K, Li L, You Z (2019) PD-L1 instead of PD-1 status is associated with the clinical features in human primary prostate tumors. Am J Clin Exp Urol 7:159–169
Haffner MC, Guner G, Taheri D, Netto GJ, Palsgrove DN, Zheng Q, Guedes LB, Kim K, Tsai H, Esopi DM, Lotan TL, Sharma R, Meeker AK, Chinnaiyan AM, Nelson WG, Yegnasubramanian S, Luo J, Mehra R, Antonarakis ES, Drake CG, De Marzo AM (2018) Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am J Pathol 188:1478–1485
Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, Zoubeidi A (2015) PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 6:234–242
Calagua C, Russo J, Sun Y, Schaefer R, Lis R, Zhang Z, Mahoney K, Bubley GJ, Loda M, Taplin ME, Balk SP, Ye H (2017) Expression of PD-L1 in hormone-naive and treated prostate cancer patients receiving neoadjuvant abiraterone acetate plus prednisone and leuprolide. Clin Cancer Res 23:6812–6822
Carretero FJ, Del Campo AB, Flores-Martin JF, Mendez R, Garcia-Lopez C, Cozar JM, Adams V, Ward S, Cabrera T, Ruiz-Cabello F, Garrido F, Aptsiauri N (2016) Frequent HLA class I alterations in human prostate cancer: molecular mechanisms and clinical relevance. Cancer Immunol Immunother 65:47–59
Blades RA, Keating PJ, McWilliam LJ, George NJ, Stern PL (1995) Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology 46:681–686 ((discussion 686-687))
Seliger B, Stoehr R, Handke D, Mueller A, Ferrone S, Wullich B, Tannapfel A, Hofstaedter F, Hartmann A (2010) Association of HLA class I antigen abnormalities with disease progression and early recurrence in prostate cancer. Cancer Immunol Immunother 59:529–540
Bander NH, Yao D, Liu H, Chen YT, Steiner M, Zuccaro W, Moy P (1997) MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate 33:233–239
Naoe M, Marumoto Y, Aoki K, Fukagai T, Ogawa Y, Ishizaki R, Nakagami Y, Yoshida H, Ballo M (2002) MHC-class I expression on prostate carcinoma and modulation by IFN-gamma. Nihon Hinyokika Gakkai Zasshi 93:532–538
Martini M, Testi MG, Pasetto M, Picchio MC, Innamorati G, Mazzocco M, Ugel S, Cingarlini S, Bronte V, Zanovello P, Krampera M, Mosna F, Cestari T, Riviera AP, Brutti N, Barbieri O, Matera L, Tridente G, Colombatti M, Sartoris S (2010) IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine 28:3548–3557
Galon J, Angell HK, Bedognetti D, Marincola FM (2013) The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39:11–26
Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW (2009) Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115:3670–3679
Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM (2006) Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24:3089–3094
Jaroslawski S, Toumi M (2015) Sipuleucel-T (Provenge((R)))-autopsy of an innovative paradigm change in cancer treatment: why a single-product biotech company failed to capitalize on its breakthrough invention. BioDrugs 29:301–307
Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR (2010) Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28:1099–1105
Subudhi SK, Vence L, Zhao H, Blando J, Yadav SS, Xiong Q, Reuben A, Aparicio A, Corn PG, Chapin BF, Pisters LL, Troncoso P, Tidwell RS, Thall P, Wu CJ, Zhang J, Logothetis CL, Futreal A, Allison JP, Sharma P (2020) Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaz3577
Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, Piulats JM, Gonzalez Mella P, Ng SS, Jaeger D, Parnis FX, Franke FA, Puente J, Carvajal R, Sengelov L, McHenry MB, Varma A, van den Eertwegh AJ, Gerritsen W (2017) Randomized, double-blind, phase iii trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol 35:40–47
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409–413
Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, Rathkopf D, Morris MJ, Danila DC, Slovin SF, Carbone E, Barnett ES, Hullings M, Hechtman JF, Zehir A, Shia J, Jonsson P, Stadler ZK, Srinivasan P, Laudone VP, Reuter V, Wolchok JD, Socci ND, Taylor BS, Berger MF, Kantoff PW, Sawyers CL, Schultz N, Solit DB, Gopalan A, Scher HI (2019) Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol 5:471–478
Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, Vaishampayan U, Berger R, Sezer A, Alanko T, de Wit R, Li C, Omlin A, Procopio G, Fukasawa S, Tabata KI, Park SH, Feyerabend S, Drake CG, Wu H, Qiu P, Kim J, Poehlein C, de Bono JS (2020) Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol 38:395–405
Fakhrejahani F, Madan RA, Dahut WL, Karzai F, Cordes LM, Schlom J, Gulley JL (2017) Avelumab in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 35:159–159
Sharma P, Pachynski RK, Narayan V, Flechon A, Gravis G, Galsky MD, Mahammedi H, Patnaik A, Subudhi SK, Ciprotti M, Duan T, Saci A, Hu S, Han GC, Fizazi K (2019) Initial results from a phase II study of nivolumab (NIVO) plus ipilimumab (IPI) for the treatment of metastatic castration-resistant prostate cancer (mCRPC; CheckMate 650). J Clin Oncol 37:142–142
Boudadi K, Suzman DL, Luber B, Wang H, Silberstein J, Sullivan R, Dowling D, Harb R, Nirschl T, Dittamore RV, Carducci MA, Eisenberger MA, Haffner M, Meeker A, Eshleman JR, Luo J, Drake CG, Antonarakis ES (2017) Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol 35:5035–5035
Santegoets SJ, Stam AG, Lougheed SM, Gall H, Scholten PE, Reijm M, Jooss K, Sacks N, Hege K, Lowy I, Cuillerot JM, von Blomberg BM, Scheper RJ, van den Eertwegh AJ, Gerritsen WR, de Gruijl TD (2013) T cell profiling reveals high CD4+CTLA-4 + T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother 62:245–256
Sweeney CJ GS, Rathkopf D, et al: IMbassador250: A phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer. 2020 AACR Virtual Annual Meeting. Abstract CT014. Presented April 27. 2020.
Graff JN, Antonarakis ES, Hoimes CJ, Tagawa ST, Hwang C, Kilari D, Tije AT, Omlin AG, McDermott RS, Vaishampayan UN, Elliott A, Wu H, Kim J, Schloss C, Bono JSD (2020) Pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-199 cohorts 4–5. J Clin Oncol 38:15–15
Karzai F, VanderWeele D, Madan RA, Owens H, Cordes LM, Hankin A, Couvillon A, Nichols E, Bilusic M, Beshiri ML, Kelly K, Krishnasamy V, Lee S, Lee MJ, Yuno A, Trepel JB, Merino MJ, Dittamore R, Marte J, Donahue RN, Schlom J, Killian KJ, Meltzer PS, Steinberg SM, Gulley JL, Lee JM, Dahut WL (2018) Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer 6:141
Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, Scher HI, Chin K, Gagnier P, McHenry MB, Beer TM (2013) Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 24:1813–1821
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengelov L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR, Investigators CA (2014) Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 15:700–712
Ross AE, Hurley PJ, Tran PT, Rowe SP, Benzon B, Neal TO, Chapman C, Harb R, Milman Y, Trock BJ, Drake CG, Antonarakis ES (2020) A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis 23:184–193
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
IT: project development, data collection and management, data analysis, manuscript writing, and manuscript editing. MPB: project development, data collection and management, data analysis, manuscript writing, and manuscript editing. EJ: data analysis and manuscript editing. CM: data collection, manuscript writing, and manuscript editing. GP: project development, data collection and management, data analysis, manuscript writing, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsaur, I., Brandt, M.P., Juengel, E. et al. Immunotherapy in prostate cancer: new horizon of hurdles and hopes. World J Urol 39, 1387–1403 (2021). https://doi.org/10.1007/s00345-020-03497-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03497-1