Abstract
Purpose
To evaluate the functional outcomes as they relate to the preservation of urinary continence and sexual function after treatment with the temporarily implanted nitinol device (iTind; Medi-Tate Ltd, Israel); a novel minimally invasive treatment for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH).
Methods
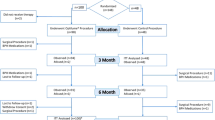
Men with symptomatic BPH (IPSS ≥ 10, Qmax < 12 ml/s, and prostate volume (PV) < 120 ml) were invited to participate in this single-arm, prospective multicenter study (MT06). Patients were not washed out of BPH medications before the procedure. The iTind was implanted through a 22F rigid cystoscope under intravenous sedation and was removed 5–7 days later through a 22F Foley catheter under local anesthesia. Post-operative VAS and complications (Clavien Dindo-Grading System) were recorded. Preservation of urinary continence and erectile and ejaculatory function were assessed according to ISI, MSHQ-EjD and SHIM questionnaires. Post-operative IPSS, QoL, Qmax and PVR were also assessed at 1, 3, and 6 months post-operatively.
Results
This interim report includes data out to 6 months on the first 70 patients enrolled in the study. The median age was 62.31 years, and the mean prostate volume was 37.68 ml (15–80 ml). Baseline and follow-up data are reported in Table 1. No intraoperative complications were observed, the average post-operative VAS score was 3.24 ± 2.56. On average patients returned to daily life after 4.3 days following the retrieval procedure. Sexual function and urinary continence were preserved in all subjects according to the ISI, SHIM and MSHQ-EjD questionnaires and significant improvements (p < 0.0001) from baseline levels were recorded in IPSS, QoL and peak flow.
Conclusion
iTind is a well-tolerated, minimally invasive treatment for BPH-related LUTS which preserves sexual function and urinary continence, offers a rapid recovery and return to daily life, and a significant improvement of symptoms and urinary flow at 6-month follow-up.
Similar content being viewed by others
References
Gratzke C, Bachmann A, Descazeaud A et al (2015) EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 67:1099–1109
Vuichoud C, Loughlin KR (2015) Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol 22(Suppl 1):1–6
De Nunzio C, Lombardo R, Gacci M et al (2015) The diagnosis of benign prostatic obstruction: validation of the young academic urologist clinical nomogram. Urology. https://doi.org/10.1016/j.urology.2015.08.003
Amparore D, De Cillis S, Volpi G et al (2019) First- and second-generation temporary implantable nitinol devices as minimally invasive treatments for BPH-related LUTS: systematic review of the literature. Curr Urol Rep. https://doi.org/10.1007/s11934-019-0912-6
Rieken M, Presicce F, Autorino R, Nunzio DEC (2017) Clinical significance of intravesical prostatic protrusion in the management of benign prostatic enlargement: a systematic review and critical analysis of current evidence. Minerva Urol Nefrol 69:548–555. https://doi.org/10.23736/S0393-2249.17.02828-4
Madersbacher S, Marberger M (1999) Is transurethral resection of the prostate still justified? BJU Int 83(3):227–237
De Nunzio C, Lombardo R, Nacchia A et al (2018) Young Academic Urologists’ benign prostatic obstruction nomogram predicts clinical outcome in patients treated with transurethral resection of prostate: an Italian cohort study. Minerva Urol Nefrol 70:211–217. https://doi.org/10.23736/S0393-2249.17.03008-9
De Nunzio C, Lombardo R, Gacci M et al (2017) Metabolic syndrome does not increase the risk of ejaculatory dysfunction in patients with lower urinary tract symptoms and benign prostatic enlargement: an Italian single-center cohort study. Urology. https://doi.org/10.1016/j.urology.2017.04.007
Lombardo R, Andersson KE, Tubaro A, De Nunzio C (2018) Intraprostatic injections for lower urinary tract symptoms/benign prostatic enlargement treatment. Minerva Urol e Nefrol 70:570–578
Cacciamani GE, Cuhna F, Tafuri A et al (2019) Anterograde ejaculation preservation after endoscopic treatments in patients with bladder outlet obstruction: systematic review and pooled-analysis of randomized clinical trials. Minerva Urol Nefrol 71:427–434. https://doi.org/10.23736/S0393-2249.19.03588-4
Rapisarda S, Russo GI, Osman NI et al (2019) The use of laser as a therapeutic modality as compared to TURP for the small prostate ≤ 40ml: a collaborative review. Minerva Urol Nefrol. https://doi.org/10.23736/S0393-2249.19.03350-2
Emberton M (2010) Medical treatment of benign prostatic hyperplasia: physician and patient preferences and satisfaction. Int J Clin Pract 64:1425–1435. https://doi.org/10.1111/j.1742-1241.2010.02463.x
Sountoulides P, Karatzas A, Gravas S (2019) Current and emerging mechanical minimally invasive therapies for benign prostatic obstruction. Ther Adv Urol 11:1756287219828971. https://doi.org/10.1177/1756287219828971
Yu X, Elliott SP, Wilt TJ, McBean AM (2008) Practice patterns in benign prostatic hyperplasia surgical therapy: the dramatic increase in minimally invasive technologies. J Urol 180:241–245. https://doi.org/10.1016/j.juro.2008.03.039 (discussion 245)
Porpiglia F, Fiori C, Amparore D et al (2019) Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. BJU Int 123:1061–1069. https://doi.org/10.1111/bju.14608
Smith C, Craig P, Taleb S et al (2017) Comparison of traditional and emerging surgical therapies for lower urinary tract symptoms in men: a review. Cardiovasc Intervent Radiol 40:1176–1184. https://doi.org/10.1007/s00270-017-1575-7
Porpiglia F, Fiori C, Bertolo R et al (2018) 3-Year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int 122:106–112. https://doi.org/10.1111/bju.14141
Bertolo R, Fiori C, Amparore D, Porpiglia F (2018) Follow-up of temporary implantable nitinol device (TIND) implantation for the treatment of BPH: a systematic review. Curr Urol Rep. https://doi.org/10.1007/s11934-018-0793-0
De Nunzio C, Lombardo R, Autorino R et al (2013) Contemporary monopolar and bipolar transurethral resection of the prostate: prospective assessment of complications using the Clavien system. Int Urol Nephrol 45:951–959. https://doi.org/10.1007/s11255-013-0476-1
Presicce F, Denunzio C, Gacci M et al (2017) The influence of the medical treatment of LUTS on benign prostatic hyperplasia surgery: Do we operate too late? Minerva Urol e Nefrol. https://doi.org/10.23736/S0393-2249.16.02815-0
Gacci M, Corona G, Sebastianelli A et al (2016) male lower urinary tract symptoms and cardiovascular events: a systematic review and meta-analysis. Eur Urol 70:788–796. https://doi.org/10.1016/j.eururo.2016.07.007
De Monte C, Carradori S, Granese A et al (2014) Modern extraction techniques and their impact on the pharmacological profile of Serenoa repens extracts for the treatment of lower urinary tract symptoms. BMC Urol. https://doi.org/10.1186/1471-2490-14-63
Kluivers KB, Riphagen I, Vierhout ME et al (2008) Systematic review on recovery specific quality-of-life instruments. Surgery 143:206–215. https://doi.org/10.1016/j.surg.2007.08.017
Marcon J, Magistro G, Stief CG, Grimm T (2018) What’s new in TIND? Eur Urol Focus 4:40–42. https://doi.org/10.1016/j.euf.2018.04.009
Cacciamani GE, Cuhna F, Tafuri A et al (2019) Anterograde ejaculation preservation after endoscopic treatments in patients with bladder outlet obstruction: systematic review and pooled-analysis of randomized clinical trials. Minerva Urol Nefrol. https://doi.org/10.23736/S0393-2249.19.03588-4
Funding
The study was sponsored by Medi-Tate.
Author information
Authors and Affiliations
Contributions
CDN: project development, data management, data analysis, manuscript writing and editing. FC: project development, data management, data analysis, manuscript writing and editing. CF: data collection and management and manuscript editing. FC: data collection and management, and manuscript editing. PT: protocol development, data management, data analysis, manuscript writing and editing. DA: protocol development, data management and manuscript editing. VB: protocol development, data management and manuscript editing. JRE: protocol development, data management, manuscript writing and editing. FGS: protocol development, data management, manuscript writing and editing. FP: protocol development, data management, data analysis, manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Nunzio, C., Cantiello, F., Fiori, C. et al. Urinary and sexual function after treatment with temporary implantable nitinol device (iTind) in men with LUTS: 6-month interim results of the MT-06-study. World J Urol 39, 2037–2042 (2021). https://doi.org/10.1007/s00345-020-03418-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03418-2