Abstract
Background
Lower urinary tract symptoms due to benign prostate enlargement (LUTS/BPE) can lead to significant disturbances to health-related quality of life (HRQoL) and psychological well-being. The aim of this study was to evaluate the effect of pharmacological treatment of LUTS/BPE on disease specific and generic QOL measures.
Methods
Evolution was a European prospective, multicenter multi-national, observational registry collecting real-life clinical data over 2 years on the management of LUTS/BPE in primary and secondary care. This study investigated disease-specific QOL using questionnaires such as IPSS Q8, BPH Impact Index (BII) and generic QOL using questionnaires like EuroQOL Five Dimension (EQ5D) which encompassed EQ5D VAS and EQ5D health index.
Results
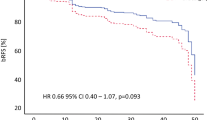
The registry enrolled 1838 BPE patients and 1246 patients were evaluable at the end of 24 months. Nearly 70% of patients in the study were previously treated with medical therapy and 17% of these had already discontinued medical treatment previously for various reasons with lack of efficacy being the most common. The mean time since diagnosis of LUTS in the previously treated group was 4.7 years (0–26 years). Medical management produced statistically significant improvement in QOL (disease specific and generic) in previously untreated patients and an insignificant change in generic QOL in previously treated patients.
Conclusions
After 5-years from the onset of symptoms, LUTS/BPE patients previously treated with medication had significantly impaired QOL in patients in a manner comparable to other chronic diseases. Earlier intervention with minimally invasive surgical techniques (MIT) should be considered in LUTS/BPE patients that do not show a significant improvement in QOL with medical therapy.




Similar content being viewed by others
References
Roehrborn CG (2011) Male Lower Urinary Tract Symptoms (LUTS) and Benign Prostatic Hyperplasia (BPH). Med Clin 95(1):87–100. https://doi.org/10.1016/j.mcna.2010.08.013
Chevalier J, de Pouvourville G (2013) Valuing EQ‐5D using time trade‐off in France. Eur J Health Econ 14(1):57–66
Pinto JDO, He H-G, Chan SWC, Wang W (2016) Health-related quality of life and psychological well-being in men with benign prostatic hyperplasia: An integrative review. Japan J Nurs Sci 13(3):309–323. https://doi.org/10.1111/jjns.12115
Hong SJ, Rayford W, Valiquette L, Emberton M (2005) The importance of patient perception in the clinical assessment of benign prostatic hyperplasia and its management. BJU Int 95(1):15–19
Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L et al (2013) Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 22(7):1717–27. https://www.ncbi.nlm.nih.gov/pubmed/23184421
Urology Sciences Research Foundation. International Prostate Symptom Score (IPSS) form [Internet]. URSF [cited 2019 Dec 26]. Available from http://www.urospec.com/uro/Forms/ipss.pdf
Angalakuditi M, Seifert RF, Hayes RP, O’Leary MP, Viktrup L (2010) Measurement properties of the benign prostatic hyperplasia impact index in tadalafil studies. Health Qual Life Outcomes 8:131. https://www.ncbi.nlm.nih.gov/pubmed/21073697
Black L, Grove A, Morrill B (2009) The psychometric validation of a US English satisfaction measure for patients with benign prostatic hyperplasia and lower urinary tract symptoms. Health Qual Life Outcomes 7:55. https://www.ncbi.nlm.nih.gov/pubmed/19545384
2019 surveillance of lower urinary tract symptoms in men: management (NICE guideline CG97). National Institute for Health and Care Excellence (UK), London; 2019. https://www.ncbi.nlm.nih.gov/books/NBK552248/
De Nunzio C, Presicce F, Lombardo R, Trucchi A, Bellangino M, Tubaro A et al (2018) Patient centred care for the medical treatment of lower urinary tract symptoms in patients with benign prostatic obstruction: a key point to improve patients’ care—a systematic review. BMC Urol 18(1):62. https://doi.org/10.1186/s12894-018-0376-x
Hutchison A, Farmer R, Verhamme K, Berges R, Navarrete RV (2007) The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries. Eur Urol 51(1):207–16. https://www.sciencedirect.com/science/article/pii/S0302283806007214
Hutchison A, Farmer R, Chapple C, Berges R, Pientka L, Teillac P et al (2006) Characteristics of patients presenting with LUTS/BPH in six European countries. Eur Urol 50(3):555–561 (discussion 562)
Fourcade R-O, Lacoin F, Rouprêt M, Slama A, Le Fur C, Michel E et al (2012) Outcomes and general health-related quality of life among patients medically treated in general daily practice for lower urinary tract symptoms due to benign prostatic hyperplasia. World J Urol 30(3):419–26. https://www.ncbi.nlm.nih.gov/pubmed/21892656
Sønksen J, Barber NJ, Speakman MJ, Berges R, Wetterauer U, Greene D et al (2015) Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol 68(4):643–652. https://doi.org/10.1016/j.eururo.2015.04.024
Perera M, Roberts MJ, Doi SAR, Bolton D (2015) Prostatic urethral lift improves urinary symptoms and flow while preserving sexual function for men with benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Urol 67(4):704–13. https://www.sciencedirect.com/science/article/pii/S0302283814011208
Funding
The Evolution registry was supported by a restricted Grant from GlaxoSmithKline© (GSK).
Author information
Authors and Affiliations
Contributions
NRB: Data analysis and Manuscript writing. NFD: Data analysis and Manuscript editing and critical review. WPW: Project development, Data Collection, Manuscript editing and critical review. AB: Project development, Data Collection, Manuscript editing and critical review. CC: Project development, Data Collection, Manuscript editing and critical review. AP: Project development, Data Collection, Manuscript editing and critical review. AT: Project development, Data Collection, Manuscript editing and critical review. AT: Project development, Data Collection, Manuscript editing and critical review.
Corresponding author
Ethics declarations
Conflict of interest
None.
Research involving human participants and/or animals
Registry data, not research.
Informed consent
Registry data, ethics obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
About this article
Cite this article
Bhatt, N.R., Davis, N.F., Witjes, W.P. et al. Quality of life with pharmacological treatment in patients with benign prostatic enlargement: results from the Evolution European Prospective Multicenter Multi-National Registry Study. World J Urol 39, 517–526 (2021). https://doi.org/10.1007/s00345-020-03219-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03219-7