Abstract
Purpose
The aim of this study was to assess the impact of experience on the outcome of image fusion-guided prostate biopsies performed by urologists working at a high-volume medical center.
Methods
The first 210 consecutive fusion biopsies were analyzed following installation of the software-based biopsy platform Artemis™ (Eigen, USA). The impact of training was measured in terms of changes in prostate cancer detection rates and biopsy duration over time. We sought to identify a threshold of experience for urologists, which predicts higher detection rates of targeted biopsies. The influence of various factors on prostate cancer detection rates was evaluated using bi- and multivariate analysis.
Results
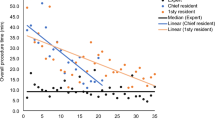
Twenty-two urologists (n = 9 senior urologists, n = 13 urological residents) performed targeted biopsies followed by systematic 12-core biopsies. Overall, targeted biopsies yielded a positive result in 39.6% of 260 suspicious MRI lesions. A subgroup analysis of the six urologists who performed more than ten biopsies was then conducted, and their level of experience (i.e., performance of more than eight biopsies) was found to be associated with higher detection rates than those with less experience (49.0% and 23.0%, respectively; p < 0.001) in the targeted biopsies. Experience was likewise a significant and independent predictor of a cancer-positive targeted biopsy (p = 0.002). Experienced senior physicians did not outperform residents in their targeted biopsy results. Further, biopsy duration correlated negatively (r = − 0.5931, p < 0.001) with the total number of biopsies performed for all subgroups during the period of assessment.
Conclusions
Experience is an important predictor of the rate of detection in targeted biopsies using software-based biopsy platforms with semi-robotic assistance. Moreover, the performance of just a few procedures appears sufficient to increase biopsy effectiveness significantly. Lastly, supervision by experts is recommended during the training phase.

Similar content being viewed by others
References
Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM (2011) Complications after prostate biopsy: data from SEER-Medicare. J Urol 186(5):1830–1834. https://doi.org/10.1016/j.juro.2011.06.057
Karam JA, Shulman MJ, Benaim EA (2004) Impact of training level of urology residents on the detection of prostate cancer on TRUS biopsy. Prostate Cancer Prostatic Dis 7(1):38–40. https://doi.org/10.1038/sj.pcan.4500695
Benchikh El Fegoun A, El Atat R, Choudat L, El Helou E, Hermieu JF, Dominique S, Hupertan V, Ravery V (2013) The learning curve of transrectal ultrasound-guided prostate biopsies: implications for training programs. Urology 81(1):12–15. https://doi.org/10.1016/j.urology.2012.06.084
Bjurlin MA, Mendhiratta N, Wysock JS, Taneja SS (2016) Multiparametric MRI and targeted prostate biopsy: improvements in cancer detection, localization, and risk assessment. Cent Eur J Urol 69(1):9–18. https://doi.org/10.5173/ceju.2016.734
Woo S, Suh CH, Kim SY, Cho JY, Kim SH (2017) Diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: a systematic review and diagnostic meta-analysis. Eur Urol 72(2):177–188. https://doi.org/10.1016/j.eururo.2017.01.042
de Rooij M, Hamoen EH, Futterer JJ, Barentsz JO, Rovers MM (2014) Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. Am J Roentgenol 202(2):343–351. https://doi.org/10.2214/AJR.13.11046
Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, Dorey FJ, Marks LS (2013) Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol 189(1):86–91. https://doi.org/10.1016/j.juro.2012.08.095
van der Kwast TH, Lopes C, Santonja C, Pihl CG, Neetens I, Martikainen P, Di Lollo S, Bubendorf L, Hoedemaeker RF, Members of the pathology committee of the European Randomised Study of Screening for Prostate C (2003) Guidelines for processing and reporting of prostatic needle biopsies. J Clin Pathol 56(5):336–340
Deutsche Gesellschaft für Urologie e.V. (DGU) (2018) Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms. Available from: http://www.awmf.org/uploads/tx_szleitlinien/043-022OLl_S3_Prostatakarzinom_2018-04.pdf. Accessed 08 Apr 2018
European Association of Urology (EAU) (2017) Guidelines on prostate cancer. Available from: http://uroweb.org/guideline/prostate-cancer/. Accessed 08 April 2018
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP, Oldroyd R, Parker C, Emberton M, Group Ps (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389(10071):815–822. https://doi.org/10.1016/S0140-6736(16)32401-1
Elkhoury FF, Simopoulos DN, Marks LS (2018) Targeted Prostate Biopsy in the Era of Active Surveillance. Urology 112:12–19. https://doi.org/10.1016/j.urology.2017.09.007
Wegelin O, van Melick HH, Hooft L, Bosch JL, Reitsma HB, Barentsz JO, Somford DM (2016) Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. https://doi.org/10.1016/j.eururo.2016.07.041
Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, Melamed J, Taneja SS (2014) A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol 66(2):343–351. https://doi.org/10.1016/j.eururo.2013.10.048
Natarajan S, Marks LS, Margolis DJ, Huang J, Macairan ML, Lieu P, Fenster A (2011) Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol 29(3):334–342. https://doi.org/10.1016/j.urolonc.2011.02.014
Sonn GA, Margolis DJ, Marks LS (2014) Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urol Oncol 32(6):903–911. https://doi.org/10.1016/j.urolonc.2013.08.006
Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, Reiter RE, Marks LS (2016) Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 122(6):884–892. https://doi.org/10.1002/cncr.29874
Baco E, Rud E, Eri LM, Moen G, Vlatkovic L, Svindland A, Eggesbo HB, Ukimura O (2016) A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol 69(1):149–156. https://doi.org/10.1016/j.eururo.2015.03.041
Meng X, Rosenkrantz AB, Mendhiratta N, Fenstermaker M, Huang R, Wysock JS, Bjurlin MA, Marshall S, Deng FM, Zhou M, Melamed J, Huang WC, Lepor H, Taneja SS (2016) Relationship between prebiopsy multiparametric magnetic resonance imaging (MRI), biopsy indication, and MRI-ultrasound fusion-targeted prostate biopsy outcomes. Eur Urol 69(3):512–517. https://doi.org/10.1016/j.eururo.2015.06.005
Delongchamps NB, Lefevre A, Bouazza N, Beuvon F, Legman P, Cornud F (2015) Detection of significant prostate cancer with magnetic resonance targeted biopsies–should transrectal ultrasound-magnetic resonance imaging fusion guided biopsies alone be a standard of care? J Urol 193(4):1198–1204. https://doi.org/10.1016/j.juro.2014.11.002
Martorana E, Pirola GM, Scialpi M, Micali S, Iseppi A, Bonetti LR, Kaleci S, Torricelli P, Bianchi G (2017) Lesion volume predicts prostate cancer risk and aggressiveness: validation of its value alone and matched with prostate imaging reporting and data system score. BJU Int 120(1):92–103. https://doi.org/10.1111/bju.13649
Okotie OT, Roehl KA, Han M, Loeb S, Gashti SN, Catalona WJ (2007) Characteristics of prostate cancer detected by digital rectal examination only. Urology 70(6):1117–1120. https://doi.org/10.1016/j.urology.2007.07.019
Calio B, Sidana A, Sugano D, Gaur S, Jain A, Maruf M, Xu S, Yan P, Kruecker J, Merino M, Choyke P, Turkbey B, Wood B, Pinto P (2017) Changes in prostate cancer detection rate of MRI-TRUS fusion vs systematic biopsy over time: evidence of a learning curve. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/pcan.2017.34
Mager R, Brandt MP, Borgmann H, Gust KM, Haferkamp A, Kurosch M (2017) From novice to expert: analyzing the learning curve for MRI-transrectal ultrasonography fusion-guided transrectal prostate biopsy. Int Urol Nephrol 49(9):1537–1544. https://doi.org/10.1007/s11255-017-1642-7
Gaziev G, Wadhwa K, Barrett T, Koo BC, Gallagher FA, Serrao E, Frey J, Seidenader J, Carmona L, Warren A, Gnanapragasam V, Doble A, Kastner C (2016) Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int 117(1):80–86. https://doi.org/10.1111/bju.12892
Rosenkrantz AB, Ayoola A, Hoffman D, Khasgiwala A, Prabhu V, Smereka P, Somberg M, Taneja SS (2017) The learning curve in prostate MRI Interpretation: self-directed learning versus continual reader feedback. Am J Roentgenol 208(3):W92–W100. https://doi.org/10.2214/AJR.16.16876
Shah MD, Parwani AV, Zynger DL (2017) Impact of the pathologist on prostate biopsy diagnosis and immunohistochemical stain usage within a single institution. Am J Clin Pathol 148(6):494–501. https://doi.org/10.1093/ajcp/aqx103
Author information
Authors and Affiliations
Contributions
Study concept and design: NW, MCK, MR; Acquisition of data: HH, NW, JB, SP; Analysis and interpretation of data: NW, HH, MCK, MR; Drafting of the manuscript: NW; Critical revision of the manuscript: MCK, JH, JB, SP, MSM, PH, MR; Statistical analysis: NW, MCK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
This study was approved by the institutional ethical review board (2018-878R-MA).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Westhoff, N., Haumann, H., Kriegmair, M.C. et al. Association of training level and outcome of software-based image fusion-guided targeted prostate biopsies. World J Urol 37, 2119–2127 (2019). https://doi.org/10.1007/s00345-018-2605-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2605-z