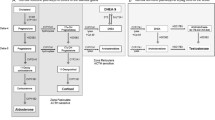
Abstract
Accumulating evidence has shown that intracrinology in prostate cancer (PCa) has a pivotal role in survival of cancer cell. PCa cells are able to produce androgens from different androgen precursors, such as dehydroepiandrosterone, thereby maintaining androgen receptor signaling. Several drugs have been developed that target intracrinology, some of which are now being used as standard treatment for the so-called castrate-resistant prostate cancer (CRPC) patients. Recently, the US FDA approval has changed the indication of drugs targeting intracrinology, e.g., abiraterone and enzalutamide where it evolved from post-chemotherapy CRPC to hormone-naive metastatic PCa cases. This approval raises question whether those drugs can also be used as the first-line treatment in localized stage PCa cases. In addition, development of additional drugs targeting major components of intracrinology is ongoing. Application of these new drugs and administration of combinations of existing drugs will ultimately lead to an increase in the efficacy of such treatments as well as to reduce the toxicity of the therapy and to prevent the risk of resistance.




Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Center MM, Jemal A, Lortet-Tiulent J et al (2012) International variation in prostate cancer incidence and mortality rates. Eur Urol 61(6):1079–1082
Alva A, Hussain M (2013) The changing natural history of metastatic prostate cancer. Cancer J 19(1):19–24
Perlmutter MA, Lepor H (2007) Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol 9(Suppl 1):S3–S8
Chandrasekar T, Yang JC, Gao AC, Evans CP (2015) Mechanism of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol 4(3):365–380
Chi KN, Bjartell A, Dearnaley D et al (2009) Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol 56(4):594–605
Labrie F (2015) Combined blockade of testicular and locally made androgens in prostate cancer: a highly significant medical progress based upon intracrinology. J Steroid Biochem Mol Biol 145C:144–156
Harris WP, Mostaghel EA, Nelson PS, Montgomery B (2009) Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol 6(2):76–85
Mostaghel EA, Nelson PS (2008) Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab 22(2):243–258
Sharifi N (2012) The 5α-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer. J Investig Med 60(2):504–507
Fukami M, Homma K, Hasegawa T, Ogata T (2013) Backdoor pathway for dihydrostestosterone biosynthesis: implications for normal and abnormal human sex development. Dev Dyn 242(4):320–329
Chang KH, Li R, Papari-Zareei M et al (2011) Dihydrostestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci USA 108(33):13728–13733
Liedtke AJ, Adeniji AO, Chen M et al (2013) Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem 56(6):2429–2446
Fizazi K, Scher HI, Molina A et al (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13(10):983–992
Guo C, Yeh S, Niu Y et al (2017) Targeting androgen receptor versus targeting androgens to suppress castration resistant prostate cancer. Cancer Lett 1(397):133–143
Hamid AR, Verhaegh GW, Smit FP et al (2015) Dutasteride and enzalutamide synergistically suppress prostate tumor cell proliferation. J Urol 193(3):1023–1029
Parker C, Gillessen S, Heidenerich A, Horwich A (2015) Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v69–v77
Kluetz PG, Ning YM, Maher VE, Zhang L, Tang S, Ghosh D (2013) Abiraterone acetate in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer: U.S. food and drug administration drug approval summary. Clin Cancer Res 19(24):6650–6656
Ning YM, Brave M, Maher VE, Zhang L, Tang S, Sridhara RUS (2015) Food and drug administration approval summary: enzalutamide for the treatment of patients with chemotherapy-naive metastatic castration-resistant prostate cancer. Oncologist 20:960–966
Fizazi K, Tran N, Fein L et al (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352–360
James ND, Bono JS, Spears MR et al (2017) Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 377:338–351
Scher HI, Fizazi K, Saad F et al (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367(13):1187–1197
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN (2015) Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, doube-blind, placebo-controlled phase 3 study. Lancet Oncol 16(2):152–160
Beer TM, Armstrong AJ, Rathkopf DE et al (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424–433
Tombal B, Borre M, Rathenborg P, Werbrouck P, Poppel HV, Heidenreich A (2014) Enzalutamide monotherapy in hormone-naive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol 15:592–600
Liu C, Lou W, Zhu Y, Yang JC, Nadiminy N, Nilesh W (2015) Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res 75(7):1413–1422
Liu C, Armstrong CM, Lou W, Lombard A, Christopher PE, Gao AC (2017) Inhibition of AKR1C3 activation overcomes resistance to abiraterone in advanced prostate cancer. Mol Cancer Ther 16(1):35–44
Schweizer MT, Yu EY (2016) Targeting intratumoral androgens: statins and beyond. Ther Adv Med Oncol 8(5):388–395
Terakawa T, Katsuta E, Yan L et al (2018) High expression of SLCO2B1 is associated with prostate cancer recurrence after radical prostatectomy. Oncotarget 9(18):14207–14218
Harshman L, Wang X, Nakabayashi M et al (2015) Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol 1:495–504
Sekine Y, Nakayama H, Miyazawa Y et al (2018) Simvastatin in combination with meclofenamic acid inhibits the proliferation and migration of human prostate cancer PC-3 cells via an AKR1C3 mechanism. Oncol Lett 15(3):3167–3172
Burska UL, Harle VJ, Coffey K et al (2013) Deubiquitinating enzyme Usp12 is a novel co-activator of the androgen receptor. J Biol Chem 288(45):32641–32650
McClurg UL, Azizyan M, Dransfield DT et al (2018) The novel anti-androgen candidate galeterone targets deubiquitinating enzymes, USP12 and USP46 to control prostate cancer growth and survival. Oncotarget 9(38):24992–25007
Yamashita S, Lai KP, Chuang KL et al (2012) ASC-J9 suppresses castration-resistang prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia 14(1):74–83
Boudadi K, Antonarakis ES (2016) Resistance to novel antiandrogen therapies in metastatic castration-resistand prostate cancer. Clin Med Insights Oncol 10(Suppl 1):1–9
Attard G, Sydes MR, Mason MD et al (2014) Combining enzalutamide with abiraterone, prednisone, and androgen deprivation therapy in the STAMPEDE trial. Eur Urol 66:799–802
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Rights and permissions
About this article
Cite this article
Hamid, A.R.A.H., Tendi, W., Sesari, S.S. et al. The importance of targeting intracrinology in prostate cancer management. World J Urol 37, 751–757 (2019). https://doi.org/10.1007/s00345-018-2529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2529-7