Abstract
Purpose
To optimize the rescreening schedule for men with low baseline prostate-specific antigen (PSA) levels, we evaluated men with baseline PSA levels of ≤1.0 ng/mL in PSA-based population screening.
Methods
We enrolled 8086 men aged 55–69 years with baseline PSA levels of ≤1.0 ng/mL, who were screened annually. The relationships of baseline PSA and age with the cumulative risks and clinicopathological features of screening-detected cancer were investigated.
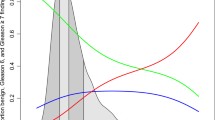
Results
Among the 8086 participants, 28 (0.35 %) and 18 (0.22 %) were diagnosed with prostate cancer and cancer with a Gleason score (GS) of ≥7 during the observation period, respectively. The cumulative probabilities of prostate cancer at 12 years were 0.42, 1.0, 3.4, and 4.3 % in men with baseline PSA levels of 0.0–0.4, 0.5–0.6, 0.7–0.8, and 0.9–1.0 ng/mL, respectively. Those with GS of ≥7 had cumulative probabilities of 0.42, 0.73, 2.8, and 1.9 %, respectively. The cumulative probabilities of prostate cancer were significantly lower when baseline PSA levels were 0.0–0.6 ng/mL compared with 0.7–1.0 ng/mL. Prostate cancer with a GS of ≥7 was not detected during the first 10 years of screening when baseline PSA levels were 0.0–0.6 ng/mL and was not detected during the first 2 years when baseline PSA levels were 0.7–1.0 ng/mL.
Conclusions
Our study demonstrated that men with baseline PSA levels of 0.0–0.6 ng/mL might benefit from longer screening intervals than those recommended in the guidelines of the Japanese Urological Association. Further investigation is needed to confirm the optimal screening interval for men with low baseline PSA levels.

Similar content being viewed by others
References
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Páez A, Määttänen L, Bangma CH, Aus G, Carlsson S, Villers A, Rebillard X, van der Kwast T, Kujala PM, Blijenberg BG, Stenman UH, Huber A, Taari K, Hakama M, Moss SM, de Koning HJ, Auvinen A, Investigators ERSPC (2012) Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 366(11):981–990
Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, Pihl CG, Stranne J, Holmberg E, Lilja H (2013) Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 11(8):725–732
Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, Holmberg L, Kantoff P, Konety BR, Murad MH, Penson DF, Zietman AL (2013) Early detection of prostate cancer: AUA guideline. J Urol 190(2):419–426
Bouchardy C, Fioretta G, Rapiti E, Verkooijen HM, Rapin CH, Schmidlin F, Miralbell R, Zanetti R (2008) Recent trends in prostate cancer mortality show a continuous decrease in several countries. Int J Cancer 123(2):421–429
Sawada K, Kitagawa Y, Ito K, Takeda Y, Mizokami A, Namiki M (2014) Cumulative risk of developing prostate cancer in men with low (≤2.0 ng/mL) prostate-specific antigen levels: a population-based screening cohort study in Japan. Int J Urol 21(6):560–565
Aus G, Damber JE, Khatami A, Lilja H, Stranne J, Hugosson J (2005) Individualized screening interval for prostate cancer based on prostate-specific antigen level: results of a prospective, randomized, population-based study. Arch Intern Med 165(16):1857–1861
Ito K, Raaijmakers R, Roobol M, Wildhagen M, Yamanaka H, Schröder FH (2005) Prostate carcinoma detection and increased prostate-specific antigen levels after 4 years in Dutch and Japanese males who had no evidence of disease at initial screening. Cancer 103(2):242–250
Roobol MJ, Roobol DW, Schröder FH (2005) Is additional testing necessary in men with prostate-specific antigen levels of 1.0 ng/ml or less in a population-based screening setting? (ERSPC, section Rotterdam). Urology 65(2):343–346
Candas B, Labrie F, Gomez JL, Cusan L, Chevrette E, Lévesque J, Brousseau G (2006) Relationship among initial serum prostate specific antigen, prostate specific antigen progression and prostate cancer detection at repeat screening visits. J Urol 175(2):510–517
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology (2014) European Association of Urology: EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65(1):124–137
Committee for Establishment of the Guidelines on Screening for Prostate Cancer; Japanese Urological Association (2010) Updated Japanese Urological Association Guidelines on prostate-specific antigen-based screening for prostate cancer in 2010. Int J Urol 17(10):830–838
Randazzo M, Beatrice J, Huber A, Grobholz R, Manka L, Chun FK, Kluth LA, Wyler SF, Recker F, Kwiatkowski M (2015) Is further screening of men with baseline PSA < 1 ng ml−1 worthwhile? The discussion continues-results of the Swiss ERSPC (Aarau). Int J Cancer 137(3):553–559
Kobori Y, Kitagawa Y, Mizokami A, Komatsu K, Namiki M (2008) Free-to-total prostate-specific antigen (PSA) ratio contributes to an increased rate of prostate cancer detection in a Japanese population screened using a PSA level of 2.1–10.0 ng/ml as a criterion. Int J Clin Oncol 13(3):229–232
Kitagawa Y, Mizokami A, Nakashima K, Koshida K, Shimamura M, Miyazaki K, Koyama N, Namiki M (2011) Clinical outcomes of prostate cancer patients detected by prostate-specific antigen-based population screening in Kanazawa City, Japan. Int J Urol 18(8):592–596
International Union Against Cancer (1997) Urologic tumors: prostate. In: Sobin LH, Wittekind CH (eds) TNM classification of malignant tumours, 5th edn. Wiley, New York, pp 170–173
Kitagawa Y, Sawada K, Urata S, Izumi K, Ueno S, Kadono Y, Konaka H, Mizokami A, Namiki M (2014) Impact of PSA levels on second-round screening for the development of prostate cancer in men with low baseline PSA levels (≤2.0 mg/ml). Anticancer Res 34(11):6739–6746
Krumholtz JS, Carvalhal GF, Ramos CG, Smith DS, Thorson P, Yan Y, Humphrey PA, Roehl KA, Catalona WJ (2002) Prostate-specific antigen cutoff of 2.6 ng/ml for prostate cancer screening is associated with favorable pathologic tumor features. Urology 60(3):469–473
Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA Jr (2004) Prevalence of prostate cancer among men with a prostate-specific antigen levels ≤4.0 ng per milliliter. N Engl J Med 350(22):2239–2246
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, Grading Committee ISUP (2005) The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostate carcinoma. Am J Surg Pathol 29(9):1228–1242
Ito K, Yamamoto T, Ohi M, Takechi H, Kurokawa K, Suzuki K, Yamanaka H (2003) Natural history of PSA increase with and without prostate cancer. Urology 62(1):64–69
Acknowledgments
We thank Dr. Kazuto Ito of Gunma University, Maebashi for valuable advice.
Authors’ contributions
S Urata was involved in data analysis and manuscript writing. Y Kitagawa was involved in protocol/project development, data management, and manuscript editing. S Matsuyama, R Naito, and K Yasuda collected the data. A Mizokami was involved in data collection and data management. M Namiki was involved in protocol/project development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethical standards
This study has been approved by an institutional review board for research in Kanazawa University (1558-1 Kanazawa University Hospital). There is no patient identifying information included in this manuscript.
Rights and permissions
About this article
Cite this article
Urata, S., Kitagawa, Y., Matsuyama, S. et al. Optimal screening interval for men with low baseline prostate-specific antigen levels (≤1.0 ng/mL) in a prostate cancer screening program. World J Urol 35, 579–586 (2017). https://doi.org/10.1007/s00345-016-1894-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1894-3