Abstract
Prolonged drought periods cause a cascade of biochemical alterations in plants and lead to reduction in plant growth and crop productivity. Seaweed extracts are a category of plant biostimulants that are effective in alleviating drought stress on plants. However, the effect of seaweed extracts on attenuating the negative impact of drought on Faba bean (Vicia faba L.) under arid and semi-arid conditions has received little attention. This study was conducted to evaluate the performances of extracts made from Fucus spiralis (FSE), Ulva lactuca (ULE), Laminaria ochroleuca (LOE), and Ascophyllum nodosum (ANE) in mitigating drought stress in Faba bean. The biochemical profile of the extracts was characterized, and key physiological and biochemical parameters of Faba bean plants were assessed during both drought and recovery phases. All investigated extracts positively affected plant biomass under drought stress conditions. Plants that received LOE had a higher relative water content and lower malondialdehyde concentration in comparison with stressed control plants. The positive effect of ANE and FSE was mainly attributed to proline accumulation in plant tissues under stress. FSE and ULE application resulted in a higher concentration of soluble sugars in treated plants in comparison to the control plants. It was concluded that seaweed extracts originating from different sources and extracted using different protocols act differentially in altering plant-related stress traits. Overall, seaweed extracts are potentially an effective solution to manage the negative impact of water scarcity on Faba bean.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought is a damaging natural hazard that is increasingly impacting the world’s population, especially those living in arid and semi-arid areas (FAO 2022). Soil moisture insufficiency is due to a combination of precipitation deficits, elevated vapor deficit, and high atmospheric evaporation which impacts on plant growth and crop productivity (Zhang et al. 2022).
Prolonged drought periods cause a reduction in plant growth and respiration, negatively alter the ATP synthesis, increase reactive oxygen species (ROS) production, increase the biosynthesis of enzymatic and non-enzymatic antioxidants, increase the biosynthesis of osmoregulatory compounds like proline and glycine betaine, enhance soluble sugars and proteins content, enhance lipid peroxidation and could lead to plant death (Cvikrová et al. 2013; Seleiman et al. 2021; Bondok et al. 2022). Different technologies and approaches have been developed to deal with the negative effects of drought impacting plant growth and development. Genetic engineering, biochar, plant growth regulators, plant growth promoting microorganisms, fertilizers, seed priming, and seaweed extracts are examples of such strategies (Ali et al. 2017; Hussain et al. 2018).
Seaweed extracts (SE) are an important category of plant biostimulants that proved their efficacy in alleviating drought stress on plants. They are estimated to constitute more than 33% of the global biostimulant market (EL Boukhari et al. 2020). Ascophyllum nodosum is the most widely processed algae in the SE industry (Shukla et al. 2019). Their action is mainly linked to their capacity in alleviating the oxidative damage on plants by scavenging ROS, enhancing the synthesis of enzymatic and non-enzymatic antioxidants, e.g., total phenols, equilibrating abscisic acid production, improving photosynthetic performances, enhancing electrical leakage, improving the accumulation of sugars and osmoregulators, and minimizing ionic imbalances and lipid peroxidation (Santaniello et al. 2017; Sharma et al. 2019; El Boukhari et al. 2021).
Faba bean (Vicia faba L.) is one of the most important cropped food legumes in the world. However, biotic and abiotic stresses such as drought have significantly affected its availability and productivity (FAO 2011). Drought reduces biomass and chlorophyll content of Faba bean (Siddiqui et al. 2015) and symbiotic fixation of nitrogen, nodulation, and grain and straw yields (Katerji et al. 2011). Relative water content is also reduced under drought stress conditions (Alghamdi et al. 2015). Intercropping, breeding, absorbent nanocomposites, amino acids, and growth regulators are some of the technologies that have been adopted for attenuating the negative impact of water deficit on Faba bean (Abid et al. 2020; Mansour et al. 2021; Rady et al. 2021; Meißner et al. 2021; Kenawy et al. 2022). Other innovations are still needed to further improve Faba bean production under such limiting conditions.
In this context, this study was conducted (1) to investigate the efficacy of some seaweed extracts in mitigating drought stress on Faba bean and (2) to identify mechanisms of action by which these extracts induce resistance to drought. To achieve these objectives, plants received different seaweed extracts before and after a water shortage phase. Then physiological and biochemical parameters were followed during drought and rewatering periods.
Materials and Methods
Plant Growth Conditions
Faba bean plants (Vicia faba, cv. Super Aguadulce) were sown in 2 L pots using natural soil as a medium (Table 1) on October 26th, 2021. Plants were grown in a covered shelter at the experimental farm of Mohammed VI Polytechnic University (32.21986, − 7.891537) in a completely randomized blocks design with 5 replicates (5 pots per treatment and 1 plant per pot). Environmental conditions were monitored during the experiment (Fig. 1). Plants were irrigated by watering every second day to maintain 70% water holding capacity in the soil.
Seaweed Extracts Preparation
Ulva lactuca, Laminaria ochroleuca, and Fucus spiralis seaweeds were collected from El Jadida city coastal region, Morocco (33.24210, − 8.54602) during the low tide period in August 2021. Returning to the laboratory, seaweeds were thoroughly washed with tap water to remove sand and epiphytes. Afterwards, they were oven dried at 60 °C for 72 h, ground, and sieved to obtain particles less than 1 mm of diameter.
Ulva lactuca and Laminaria ochroleuca were extracted in an acid solution of water (pH 3). Fucus spiralis was extracted in an alkaline solution (pH 10). pH was adjusted by adding whether 1 M HCl or 1 M KOH to distilled water. The choice of the extraction protocol for each species was based on some preliminary experiments (Data not shown). Mixtures (Seaweed/water solution) (1/10: g/mL) were heated in a water bath (60 °C) for 3 h before being filtered using muslin cloth. The resultant extracts were considered as 100% extract concentration. A commercial extract of Ascophyllum nodosum (Stella Maris, Acadian Plant Health) was also used. Ulva lactuca extract (ULE), Laminaria ochroleuca extract (LOE), and Fucus spiralis extract (FSE) were applied at a dilution of 5% (v/v) while Ascophyllum nodosum extract (ANE) was applied at a dilution of 2% (v/v). Control plants received water instead of seaweed extracts.
Treatment Application and Drought Stress Methodology
ULE, LOE, FSE, ANE, and control treatment (100 mL/pot) were applied as a soil drench 22 days after sowing (DAS). A second application of the treatments was done as a foliar spray (10 mL/plant) one day before applying the drought stress regime (40 DAS). Drought stress consisted of water withholding for 10 days. After the drought treatment, plants were re-irrigated (50 DAS), and 1 day later, seaweed extracts were applied as a foliar spray (10 mL/plant). This recovery phase continued for 20 days after drought to achieve 70 DAS plants. Another batch of control plants was grown in the same experimental conditions and was continuously irrigated without receiving the drought stress treatment. These plants were labeled as non-stressed control (NS control).
To evaluate the effect of seaweed extracts on alleviating drought stress, leaf samples were taken during the water withholding phase (WWP) (49 DAS) as well as during the recovery phase (RP) (69 DAS).
Determination of Seaweed Extracts Biochemical Compounds
The seaweed extracts obtained in this study as well as the commercial product were frozen dried using a freeze dryer (Labconco corporation, Missouri USA) prior to biochemical analyses. Total proteins were determined according to Bradford (1976), and values were expressed as mg bovine serum albumin (BSA).g−1 dry weight (DW). Proline was quantified using the method described by Bates et al. (1973) and was reported as µmol.g−1 DW. Soluble sugars were estimated according to Dubois et al. (1956) and values were given as mg Glucose.g−1 DW. Total phenolic compounds were determined as described by Ainsworth and Gillespie (2007) and were expressed as mg gallic acid equivalent (GAE).g−1 DW. Analyses were made in triplicates.
Plant Growth and Physiology Parameters
Faba bean plants were harvested at the end of the RP (70 DAS), and plants’ fresh weight (FW) were determined. The DW was obtained by oven-drying plants at 60 °C for 72 h. Chlorophyll index was estimated on the youngest fully expanded leaves using a CL-01 Chlorophyll Meter (Hansatech Instruments). Chlorophyll fluorescence was quantified using a plant efficiency analyzer, Handy PEA (Hansatech Instruments) on the youngest fully expanded leaves. Leaves were clipped in the middle using leaf dark clips for 20 min. The maximum quantum efficiency of PSII photochemistry (Fv/Fm) and the photosynthetic performance index (PIABS) were measured on the top leaf surface directly after the dark adaptation using a photosynthetic photon flux density of 3000 μmol m−2 s−1 as saturating flash (Sharma et al. 2015; Chtouki et al. 2022). Vegetation indices NDVI, Normalized Difference Vegetation Index; PRI, Photochemical Reflectance Index; ARI1, ARI2, Anthocyanin Reflectance Indices; and CRI1, CR2, Carotenoid Reflectance Indices of the youngest fully expanded leaves were determined using a portable handheld spectro-radiometer PolyPen RP410/UVIS (Photon Systems Instruments). The PolyPen device integrates an internal light source with radiation range 380–790 nm. Chlorophyll index, Chlorophyll fluorescence, Fv/Fm, PIABS, and vegetation indices were measured during both the WWP (48 DAS) and the RP (68 DAS). Readings were done on two leaves/plant.
Relative Water Content (RWC)
Relative water content was measured as described by González et al. (2001) at both WWP and RP. Leaves were sampled and their FW immediately weighed. Then, the leaves were imbibed overnight in distilled water in 2 mL Eppendorf tubes at 4 °C. Afterwards, leaves were recuperated, and excess water on the surface was eliminated before recording their turgid weight (TW). Finally, samples were put in an oven to determine their DW. RWC was calculated using the following equation:
Plant Samples Extraction
Leaf samples (300 mg) at both WWP (49 DAS) and RP (69 DAS) were extracted in water (3 mL) using a mortar and pestle. The mixture was placed in Eppendorf tubes and centrifuged for 5 min, 3070 g at room temperature. The supernatant was recovered and was used as plant extract for the determination of the following biochemical traits.
Total Protein
Proteins were determined according to the Bradford assay (Bradford 1976). Plant extract (0.1 mL supernatant) was added to 0.1 mL water and 2 mL Bradford reagent in a test tube. The mixture was homogenized, and the absorbance recorded at 595 nm after 1 min. BSA was used as a standard, and proteins content was given as mg equivalent BSA.g−1 FW. Measures were made in 5 replicates.
Proline
Proline content was determined according to Bates et al. (1973) with modifications. Plant extract (1 mL) was added to 1 mL glacial acetic acid and 1 mL ninhydrin acid. The homogenate was incubated for 1 h at 100 °C in a water bath. The mixture was cooled on ice before 4 mL toluene was added. After mixing thoroughly for 20 s, the toluene phase was recovered, and absorbance was read at 528 nm. Proline was used as a standard and values were given as µmol.g−1 FW. Measures were made in 5 replicates.
Soluble Sugars
Soluble sugars were quantified based on the method developed by Dubois et al. (1956). Plant extract (0.2 mL) was added to 0.2 mL phenol 5% (w/v). Next, 1 mL sulfuric acid 95.5% was added rapidly to the solution. The mixture was incubated for 10 min in a water bath at 100 °C and then cooled on ice. Absorbance was determined at 490 nm. Glucose was used as a standard solution and values were given as mg glucose.g−1 FW. Measures were made in 5 replicates.
Total Phenolic Compounds
Total phenolic compounds were determined based on the protocol described by Ainsworth et al. (2007). Folin ciocalteu reagent (230 µL of 10% (v/v)) was added to 100 µL sample or blank. Then, 800 µL 700 mM Na2CO3 was added to the homogenate. After incubating for 2 h, absorbance was read at 765 nm. The total phenolic content was expressed as mg GAE.100 g−1 FW. Measures were made in 5 replicates.
Hydrogen Peroxide (H2O2)
The hydrogen peroxide concentration was determined as described by Velikova et al. (2000). Plant extract (500 µL) was added to 500 µL 10 mM potassium phosphate buffer (pH 7.0) and 1 mL 1 M of potassium iodide. Absorbance was read at 390 nm. H2O2 was used as a standard and values were given as µmol H2O2.g−1 FW. Measures were made in 5 replicates.
Lipid Peroxidation
Lipid peroxidation was estimated by the quantification of malondialdehyde (MDA) in leaf tissues. MDA was determined as described by Chu et al. (2010) with modifications. Plant extract (1 mL) was added to 2.5 mL 0.5% TBA in 20% TCA and incubated in hot water (95 °C) for 30 min. The reaction was stopped by placing it on ice. The homogenate was centrifuged at 10,000 rpm for 30 min before reading the absorbance at 532 and 600 nm. MDA concentration was estimated by subtracting the non-specific absorption at 600 nm from the absorption at 532 nm, using an absorbance coefficient of extinction (155 mM−1 cm−1). Measures were made in 5 replicates.
Statistical Analysis
A completely randomized blocks design with one factor (seaweed extracts) and five replicates was adopted for this experiment. Data were analyzed using ANOVA followed by Tukey’s multiple tests using the Minitab 20 statistical software.
Results
Seaweed Extracts Biochemical Profile
Seaweed extracts biochemical profiling showed statistically significant differences in their compositional chemistry (Table 2). All extracts showed high concentrations of soluble sugars except for LOE. The highest value occurred in ULE. FSE and ANE had the highest concentrations of total phenols. FSE had the significantly highest proteins content and ANE, the lowest content. Proline concentration was more than sevenfold higher in ULE in comparison with other extracts.
Fresh and Dry Weights
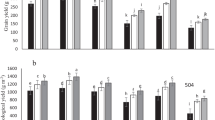
The application of drought stress (water withholding) significantly decreased fresh and dry weights of Vicia faba plants (p < 0.001) (Fig. 2). The application of ANE, FSE, LOE, and ULE had no significant effect of plant fresh weight in comparison to control plants that received drought treatment (Fig. 2A). Application of seaweed extracts enhanced plant dry weight with FSE and ANE significantly increasing the dry weight in comparison to the control plants that received drought treatment (Fig. 2B).
Fresh and Dry weights (A and B respectively) of harvested plants treated with different seaweed extracts NS Control non-stressed control, Control, FSE Fucus spiralis extract, LOE Laminaria ochroleuca extract, ANE Ascophyllum nodosum extract, and ULE Ulva lactuca extract. Different letters represent the significant differences between treatments at p ≤ 0.05. Values are mean ± SE (n = 5)
Physiological Response to Drought Stress
During drought stress (WWP), the chlorophyll index of untreated plants (control plants) significantly increased over the NS control plants (well watered). No significant difference was recorded between control and treated plants. The PIabs of FSE treatment during the WWP decreased over the NS control plants. After rewatering plants at the recovery stage, mainly all plants showed similar records for the studied physiological parameters (Table. 3).
Relative Water Content
Relative water content of Vicia faba leaves decreased significantly when the drought treatment was applied to plants (p < 0.001). The application of seaweed extracts did not induce a change in RWC in comparison with stressed control. The RWC reflected an increasing kinetic after rewatering the plants. All plants showed a similar RWC status during the RP (Fig. 3).
Total Protein and Proline Content
Water withholding did not impact protein content in stressed plants in comparison to NS control. After rewatering plants, no difference was recorded between control and SE treatments (Fig. 4).
Drought stress triggered a highly significant increase of proline in plants’ leaf tissues in comparison with NS control (p < 0.001). All SE treatments were able to increase proline in comparison to the stressed control during the same growing phase. This increase was significant (p < 0.005) for both ANE and FSE treatments, which recorded an enhancement of 82.5% and 68.25%, respectively (Fig. 5). The proline content in Vicia faba leaves decreased after rewatering. No differences were recorded between the treated plants and the control at the RP.
Soluble Sugars Content
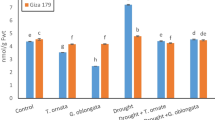
Water withholding did not induce significant reduction in soluble sugars content in comparison with the NS control. The application of ULE and FSE enabled an increase of soluble sugars content in treated plants by 16.16% and 14.22%, respectively, over the control (Fig. 6). During the RP, no significant difference was recorded among the treatments. Plants that received FSE recorded the highest value.
Total Phenols Concentration
The concentration of total phenols in Vicia faba leaves increased during drought stress application (Fig. 7). The application of FSE, ULE, and LOE reduced total phenols content of treated plants in comparison with stressed control (p > 0.05). Total phenols decreased after rewatering plants, and no statistically differences were recorded among the treatments during this RP.
Hydrogen Peroxide
Drought stress resulted in an increase of H2O2 concentration in control plants in comparison with NS control (p = 0.017). Application of ULE, ANE, and LOE induced a non-significant diminution of this concentration in treated plants by 2.2%, 4.5%, and 6.3%, respectively, in comparison with stressed control. H2O2 concentration decreased after rewatering plants. Plants which received ULE recorded the highest value at the RP. The difference between ULE and control treatment was statistically significant (p = 0.041) (Fig. 8).
Lipid Peroxidation
Lipid peroxidation was estimated based on the concentration of MDA in leaf tissues (Fig. 9). The application of drought stress caused a slight increase of MDA concentration in control plants in comparison with the well-watered plants (NS control). The application of seaweed extracts enabled the reduction of this concentration in comparison with the control during stress and recovery phases (p > 0.05). LOE significantly reduced MDA concentration at the RP in comparison to the control.
Discussion
This study confirmed that water deficit is responsible for reducing plant biomass. At the end of the recovery phase, dry biomass of stressed untreated plants had decreased by 35.83% in comparison with well-watered plants. SE application enhanced significantly DW of stressed plants by 26.11%, 25.17%, 24.55%, and 16.12% for ANE, FSE, LOE, and ULE, respectively, in comparison with the untreated stressed control at the end of the RP. Similarly, a cold extract of Gracilaria dura attenuated the effect of water withholding on wheat where 5% foliar application significantly enhanced shoot biomass by 57% in comparison with non-treated stressed control plants during the recovery stage. There was also a 70% increase in crop yield when compared to the stressed control. This enhancement was associated with a reduction in water loss and stomatal opening resulting from an increased abscisic acid (ABA) accumulation (Sharma et al. 2019).
This study showed clearly that reduction in soil water content has a negative impact on leaf RWC and consequently on plant biomass. However, LOE application increased RWC of treated plants by 19% in comparison with the stressed control during the WWP. Improving the plant water relations could be one of the major mechanisms by which SE mitigate drought on plants. For instance, orange trees irrigated under a 50% evapotranspiration (ET) regime that received a treatment of Ascophyllum nodosum extract had more new leaves. This was mainly related to the improvement of plant water relations as midday stem water potential and leaf water use efficiency. This effect was only recorded on plants that received the extract as a soil drench and not as a foliar spray (Spann et al. 2011). The positive effect on spinach leaves growth under stress after foliar and /or drench application of an Ascophyllum nodosum extract at 0.5% concentration was attributed to the improvement of leaf water relations. However, SE application did not alter chlorophyll content and fluorescence Fv/Fm of spinach plants under full irrigation and drought stress regimes (Xu et al. 2015). This was similar to the present study where the application of different SE had no effect on leaves chlorophyll index, Fv/Fm, PI abs, and NDVI during the WWP and RP.
Proline is an osmolyte that accumulates in plant cells as a response to abiotic and biotic stresses (Kavi Kishor et al. 2015). Strategies by which proline mitigates stress in plants include ROS scavenging, protection of membrane integrity, regulating cellular osmotic pressure, and maintaining enzymes /proteins stability (Dar et al. 2016). In the present study, accumulation of proline in Faba bean leaf tissues was significantly higher in plants treated with ANE and FSE in comparison with the stressed untreated control during the WWP. At the RP, proline concentrations returned to normal levels, and no difference was recorded among treatments and controls. Evidence that SEs promote the biosynthesis of proline in plant tissues during stress are well documented. For example, an application of some commercial Ascophyllum nodosum extracts significantly increased the concentration of proline in tomato leaf tissues in comparison with untreated control after a water shortage period of 7 days (Goñi et al. 2018). Other studies suggest that the application of SEs reduces the accumulation of proline in plant tissues. For instance, proline concentration of medicinal shrubs was decreased after application of an Ascophyllum nodosum extract in both 100% and 50% ET irrigation regimes (Elansary et al. 2016). Goñi et al. (2018) hypothesized that the differentiation in proline accumulation kinetics in response to SE application might be attributed to the biostimulant used, crop class, or application type. In the current study, enhanced biosynthesis of proline was recorded in all treatments regardless of the seaweed type or extraction method.
Under drought stress, sugars accumulate in plant leaves and play an important role in ROS scavenging, membrane protection, maintenance of leaves turgidity, and osmotic adjustment (Sami et al. 2016). The application of FSE and ULE enhanced the concentration of soluble sugars in Faba bean leaves in comparison to the control during stress conditions. Similarly, an application of an Ascophyllum nodosum extract as a foliar spray significantly enhanced soluble sugars concentration of vine leaves in both well-watered and stressed plants (Frioni et al. 2021). Application of Kappaphycus alvarezii extract enhanced the concentration of total soluble sugars of Triticum durum plants in both vegetative and reproductive stages under drought stress conditions (Patel et al. 2018).
The commercial product of Ascophyllum nodosum used in the current study was responsible for an increase in phenolic compounds concentration of treated plants in comparison to control plants during WWP. Similarly, a commercial SE application enhanced the concentration of total phenols of grapevine plants by more than 11% in comparison with the control during drought stress conditions (Irani et al. 2021). Phenolic compounds concentration returned to the normal at the RP, and no significant difference was recorded among treatments and controls. Phenolic compounds accumulate in plant tissues as an adaptive response to abiotic stresses. This accumulation is attributed to the activity of enzymes like phenylalanine ammonia lyase, chalcone synthase, and others (Naikoo et al. 2019).
In this study, SE application did not alter H2O2 production. However, it enabled the reduction of lipid peroxidation in treated plants, especially those that received LOE, in both WWP and RP. Similar records were reported by Mansori et al. (2016) who found that applying an Ulva rigida extract applied at concentrations greater than 25% decreased the MDA concentration in Salvia officinalis L plants under different water stress strategies. Applying an Ascophyllum nodosum extract on sugarcane induced a decrease in MDA concentration of treated plants over the control in two distinct commercial fields during drought season (Jacomassi et al. 2022).
This study revealed that the biochemical profile of extracts and the recorded positive effect on plant growth and physiological traits were not related. This suggests that the compositional molecules of the extracts are acting synergistically or other compounds that were not analyzed might be contributing to the positive effect. In a related experiment that assessed the effect of three commercial Ascophyllum nodosum extracts on alleviating drought stress on tomato, the compositional biochemistry of the extracts was not correlated to the observed improvements on tomato growth and physiology, suggesting that understanding such a relationship is complex leading to the heterogeneity of this category of biostimulants (Goñi et al. 2018).
Conclusion
The application of seaweed extracts on Faba bean plants succeeded in alleviating the negative effect of soil water limitation on plant growth and development. ANE, FSE, LOE, and ULE enabled an improvement of plant biomass under drought stress conditions. This enhancement was related mainly to the accumulation of osmoprotectants and osmoregulators as proline and soluble sugars, the improvement of relative water content in plant tissues as well as phenols, and the reduction of lipid peroxidation. In the current study, extracts like FSE showed similar performances to the commercial ANE. However, the mechanism of action by which they are acting on the alleviation of drought on Faba bean was different. This study findings demonstrate that seaweed extracts can alleviate drought stress through not a unique but multiple mechanisms, involving a variety of bioactive compounds. This suggests that the seaweed extracts manufactures must deploy additional efforts in elucidating mechanisms by which their products act on the plant soil system and do not be solely limited to their compositional biochemistry. This should broader the selection of seaweed species that might be exploited by the industry and further market opportunities.
References
Abid G, Ouertani RN, Jebara SH et al (2020) Alleviation of drought stress in faba bean (Vicia faba L.) by exogenous application of β-aminobutyric acid (BABA). Physiol Mol Biol Plants 26:1173–1186. https://doi.org/10.1007/s12298-020-00796-0
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Alghamdi SS, Al-Shameri AM, Migdadi HM et al (2015) Physiological and molecular characterization of Faba bean (Vicia faba L.) genotypes for adaptation to drought stress. J Agron Crop Sci 201:401–409. https://doi.org/10.1111/jac.12110
Ali F, Bano A, Fazal A (2017) Recent methods of drought stress tolerance in plants. Plant Growth Regul 82:363–375. https://doi.org/10.1007/s10725-017-0267-2
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bondok AET, Mousa WME, Rady AMS, Saad-Allah KM (2022) Phenotypical, physiological and molecular assessment of drought tolerance of five Egyptian teosinte genotypes. Journal of Plant Interactions 17:656–673. https://doi.org/10.1080/17429145.2022.2085335
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chtouki M, Laaziz F, Naciri R et al (2022) Interactive effect of soil moisture content and phosphorus fertilizer form on chickpea growth, photosynthesis, and nutrient uptake. Sci Rep 12:6671. https://doi.org/10.1038/s41598-022-10703-0
Chu J, Yao X, Zhang Z (2010) Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol Trace Elem Res 136:355–363. https://doi.org/10.1007/s12011-009-8542-3
Cvikrová M, Gemperlová L, Martincová O, Vanková R (2013) Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol Biochem 73:7–15. https://doi.org/10.1016/j.plaphy.2013.08.005
Dar MI, Naikoo MI, Rehman F et al (2016) Proline accumulation in plants: roles in stress tolerance and plant development. In: Khan N, Nazar R, Khan A (eds) Osmolytes and plants acclimation to changing environment: emerging omics technologies. Springer, New Delhi, pp 155–166
Drought and Agriculture, Land & Water, Food and Agriculture Organization of the United Nations, Land & Water, Food and Agriculture Organization of the United Nations. https://www.fao.org/land-water/water/drought/droughtandag/en/. Accessed 30 Jun 2022
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
El Boukhari MEM, Barakate M, Bouhia Y, Lyamlouli K (2020) Trends in seaweed extract based biostimulants: manufacturing process and beneficial effect on soil-plant systems. Plants 9:359. https://doi.org/10.3390/plants9030359
El Boukhari MEM, Barakate M, Choumani N et al (2021) Ulva lactuca extract and fractions as seed priming agents mitigate salinity stress in tomato seedlings. Plants 10:1104. https://doi.org/10.3390/plants10061104
Elansary HO, Skalicka-Woźniak K, King IW (2016) Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol Biochem 105:310–320. https://doi.org/10.1016/j.plaphy.2016.05.024
FAO (2011) On-farm conservation and mining of local Faba bean landraces for biotic and abiotic stresses in Morocco. FAO, Rome
Frioni T, VanderWeide J, Palliotti A et al (2021) Foliar vs. soil application of Ascophyllum nodosum extracts to improve grapevine water stress tolerance. Sci Hortic 277:109807. https://doi.org/10.1016/j.scienta.2020.109807
Goñi O, Quille P, O’Connell S (2018) Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol Biochem 126:63–73. https://doi.org/10.1016/j.plaphy.2018.02.024
González L, González-Vilar M (2001) Determination of relative water content. In: Reigosa Roger MJ (ed) Handbook of plant ecophysiology techniques. Springer, Netherlands, pp 207–212
Hussain HA, Hussain S, Khaliq A et al (2018) Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00393
Irani H, ValizadehKaji B, Naeini MR (2021) Biostimulant-induced drought tolerance in grapevine is associated with physiological and biochemical changes. Chem Biol Technol Agric 8:5. https://doi.org/10.1186/s40538-020-00200-9
Jacomassi LM, de Viveiros JO, Oliveira MP et al (2022) A seaweed extract-based biostimulant mitigates drought stress in sugarcane. Front Plant Sci. https://doi.org/10.3389/fpls.2022.865291
Katerji N, Mastrorilli M, Lahmer FZ et al (2011) Faba bean productivity in saline–drought conditions. Eur J Agron 35:2–12. https://doi.org/10.1016/j.eja.2011.03.001
Kavi Kishor PB, Hima Kumari P, Sunita MSL, Sreenivasulu N (2015) Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00544
Kenawy E-R, Rashad M, Hosny A et al (2022) Enhancement of growth and physiological traits under drought stress in Faba bean (Vicia faba L.) using nanocomposite. J Plant Interact 17:404–418. https://doi.org/10.1080/17429145.2022.2038293
Mansori M, Chernane H, Latique S et al (2016) Effect of seaweed extract (Ulva rigida) on the water deficit tolerance of Salvia officinalis L. J Appl Phycol 28:1363–1370. https://doi.org/10.1007/s10811-015-0671-9
Mansour E, Desoky ESM, Ali MMA et al (2021) Identifying drought-tolerant genotypes of Faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric Water Manag 247:106754. https://doi.org/10.1016/j.agwat.2021.106754
Meißner A, Granzow S, Wemheuer F, Pfeiffer B (2021) The cropping system matters—contrasting responses of winter Faba bean (Vicia faba L) genotypes to drought stress. J Plant Physiol 263:153463. https://doi.org/10.1016/j.jplph.2021.153463
Naikoo MI, Dar MI, Raghib F et al (2019) Chapter 9—Role and regulation of plants phenolics in abiotic stress tolerance: an overview. In: Khan MIR, Reddy PS, Ferrante A, Khan NA (eds) Plant signaling molecules. Woodhead Publishing, pp 157–168
Patel K, Agarwal P, Agarwal PK (2018) Kappaphycus alvarezii sap mitigates abiotic-induced stress in Triticum durum by modulating metabolic coordination and improves growth and yield. J Appl Phycol 30:2659–2673. https://doi.org/10.1007/s10811-018-1423-4
Rady MM, Boriek SHK, Abd El-Mageed TA et al (2021) Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 10:748. https://doi.org/10.3390/plants10040748
Sami F, Yusuf M, Faizan M et al (2016) Role of sugars under abiotic stress. Plant Physiol Biochem 109:54–61. https://doi.org/10.1016/j.plaphy.2016.09.005
Santaniello A, Scartazza A, Gresta F et al (2017) Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01362
Seleiman MF, Al-Suhaibani N, Ali N et al (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10:259. https://doi.org/10.3390/plants10020259
Sharma DK, Andersen SB, Ottosen C-O, Rosenqvist E (2015) Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol Plant 153:284–298. https://doi.org/10.1111/ppl.12245
Sharma S, Chen C, Khatri K et al (2019) Gracilaria dura extract confers drought tolerance in wheat by modulating abscisic acid homeostasis. Plant Physiol Biochem 136:143–154. https://doi.org/10.1016/j.plaphy.2019.01.015
Shukla PS, Mantin EG, Adil M et al (2019) Ascophyllum nodosum based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00655
Siddiqui MH, Al-Khaishany MY, Al-Qutami MA et al (2015) Response of different genotypes of Faba bean plant to drought stress. Int J Mol Sci 16:10214–10227. https://doi.org/10.3390/ijms160510214
Spann TM, Little HA (2011) Applications of a commercial extract of the brown seaweed Ascophyllum nodosum increases drought tolerance in container-grown ‘Hamlin’ sweet orange nursery trees. HortScience 46:577–582. https://doi.org/10.21273/HORTSCI.46.4.577
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Xu C, Leskovar DI (2015) Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci Hortic 183:39–47. https://doi.org/10.1016/j.scienta.2014.12.004
Zhang X, Hao Z, Singh VP et al (2022) Drought propagation under global warming: characteristics, approaches, processes, and controlling factors. Sci Total Environ 838:156021. https://doi.org/10.1016/j.scitotenv.2022.156021
Acknowledgements
The authors want to express their gratitude to all persons within the AGBS program which participated in the success of this work. Special thanks to Younes Jnaoui, El Beqqal Yassine, Jmel Aziz, and Wafa Benali for their support.
Funding
This research was supported by the Agribiotech business unit, OCP (AS40 project).
Author information
Authors and Affiliations
Contributions
MEE, KL, and MB designed the experiment, MEE and BD conducted experiments, MEE wrote the first version of the manuscript, MEE, KL, MB, BD, and YB contributed to reviewing, correcting, and validating the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Wendy Stirk.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Boukhari, M.E.M., Barakate, M., Drissi, B. et al. Seaweed Extract Biostimulants Differentially act in Mitigating Drought Stress on Faba Bean (Vicia faba L.). J Plant Growth Regul 42, 5642–5652 (2023). https://doi.org/10.1007/s00344-023-10945-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-10945-w