Abstract
Hydrogen sulfide (H2S) can alleviate Cd-induced cell death, but the molecular mechanisms are not clear. To shed light on these mechanisms, cell death induced by 200 μM cadmium chloride in cucumber seedlings root tips was used as a model system. Here, we report that the negative effect of Cd stress in mitochondrial physiological functions include changes in cytochrome c/a, mitochondrial membrane permeability transition pores, and adenosine triphosphatase (ATPase). Moreover, Cd stress led to the release of mitochondrial Ca2+ into the cytosol. Exogenous application of sodium hydrosulfide (NaHS, a donor of H2S) inhibited cell death and maintains mitochondrial function by reducing mitochondrial hydrogen peroxide accumulation, increasing ATPase activity and down-regulating CsVDAC and CsANT expression. In summary, H2S suppressed Cd-induced cell death by improving mitochondrial physiological properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium (Ca2+) as a second messenger plays a vital role in plant activity, regulation of gene expression, cell cycle control (Berridge et al. 2003), control of light signaling (Harada et al. 2003), inducing hormone responses (Shintaro et al. 2007), controlling cell growth (Sabine et al. 2007) and trigger plan responses during plant–pathogen interactions (Zhi et al. 2010). The short-term increase of cytosolic calcium ([Ca2+]Cyt) concentration is an early event in a series of biological processes and stress responses (Jörg et al. 2010; Leng 2012). After receiving intracellular or extracellular stimulation, plants activate Ca2+ channels and related transporters, induce extracellular Ca2+ influx or stored Ca2+ release to the cytosol, resulting in an instantaneous rise of Ca2+, which signals a multitude of downstream effects (Sanders et al. 2002). The Ca2+ influx and release require different Ca2+ ion channels, (i) voltage-dependent Ca2+-permeable channels (VDCCs) which are divided into depolarization-activated Ca2+-permeable channels (DACCs) and hyperpolarization-activated Ca2+-permeable channels (HACCs); (ii) voltage-independent Ca2+-permeable channels (VICCs) (Liu et al. 2018). After the completion of Ca2+ signal, in order to maintain a low concentration of Ca2+, plants need to return excess Ca2+ to the calcium stores. H+/Ca2+ antiporters and Ca2+-ATPase are the main transport and export protein, which transports Ca2+ to extracellular domain or influxes to vacuoles and mitochondria (Boursiac and Harper 2007; Jörg et al. 2010). Mitochondria are not only the energy powerhouse of the cell but also a major hub for cellular Ca2+ signaling crucial for cell life and death (Giorgi et al. 2012; Rimessi et al. 2008; Rosario et al. 2012). Mitochondria regulate cell activity by participating in dynamic regulation of Ca2+ (Marchi et al. 2018). Mitochondria, as one of the organelles storing Ca2+, play a key role in the intracellular Ca2+ signal transmission (Sisalli et al. 2012). Mitochondrial Ca2+ is involved in the process of cell death. Jones et al. found that the release of mitochondrial Cyt c into the cytoplasm induced by Ca2+ during the differentiation of tubular molecules leads to programmed cell death (PCD) (Jones 2002), and the increase of Ca2+ content in tobacco protoplasts induced the opening of mitochondrial membrane permeability, which leaded to PCD (Lin et al. 2005).
Many studies suggested that hydrogen sulfide (H2S) is an important gas signal molecule in plant, just like carbon monoxide (CO) and nitric oxide (NO) (Hancock and Whiteman 2014). L-cysteine desulfhydrase (LCD) and D-cysteine desulfhydrase (DCD) have been reported to be the key enzymes, which generate endogenous H2S (Anja et al. 2005; Riemenschneider et al. 2010). Moreover, several researches showed that H2S is involved in plant growth and development, like root development and regulating stomatal closure (Kou et al. 2018; Li et al. 2018a, 2018b; Ma et al. 2019; Chen et al. 2021), and H2S plays vital role in alleviating heavy metal stress, especially cadmium (Cd) stress (Ahmad et al. 2019; Guan et al. 2018; Zhang et al. 2015a). H2S has crosstalk with many molecules, like ethylene (Jia et al. 2018) and Ca2+. Fang et al. reported that Ca2+ in a H2S-dependent manner resisted chromium stress in millet and Ca2+/calmodulin2 (CaM2)-mediated Ca2+ signal regulated endogenous H2S content under Cr6+ stress, Cr6+ promoted the interaction between Ca2+/CAM2 and transcription factor TGA3 to enhance the expression of LCD in Arabidopsis thaliana (Fang et al. 2014a, 2017). Ca2+ activated endogenous H2S accumulation to improve thermo tolerance in tobacco suspension (Li et al. 2015). In addition, pretreatment with NaHS can improve thermo tolerance of tobacco suspension by regulating intracellular and extracellular Ca2+ concentration (Li et al. 2012). Interestingly, H2S could reduce plant cell death, like hypoxia (Cheng et al. 2013), Cd stress (Zhang et al. 2015a), and GA-triggered PCD in wheat aleurone layers (Xie et al. 2014; Zhang et al. 2015b).
The components of MPTP include voltage-dependent anion channel (VDAC), adenine nucleotide translocator (ANT), and cyclophilin D (Cyp D). ANT is located in the inner membrane of mitochondrion. It catalyzes the energy conversion between ATP and ADP of cytoplasm and mitochondria, which is very important to cells (Javadov et al. 2000). ANT is a main role in cell death. The change of mitochondrial ANT induces the degree of MPTP opening. Such as salt stress induced PCD in tobacco protoplasts (Lin et al. 2006). The VDAC is mitochondrial outer membrane components, and it is a key player in mitochondria-mediated cell death (Kusano et al. 2009). Some studies have shown that the ROS produced by VDAC overexpression was the cause of apoptosis (Huang et al. 2014; Yuan et al. 2008). VDAC and ANT might also elicit mitochondrial Ca2+ efflux since it is a part of the MPTP (Bernardi 1999; Crompton et al. 2002).
The change of intracellular Ca2+ concentration affects the state of mitochondria and leads to cell death, while H2S alleviates cell death by reducing oxidative stress. It is noteworthy that it is unclear whether H2S reduces cell death by affecting the concentration of Ca2+, especially the concentration of mitochondrial Ca2+. In this study, we investigated H2S and mitochondrial Ca2+ in terms of Cd-induced cell death, focusing on effect of H2S on mitochondrial Ca2+ transport.
Materials and Methods
Seeds of cucumber (Cucumis sativus ‘Xinchun 4’) were germinated in petri dishes linked with filter papers in darkness at 28 °C in illuminating incubation climate box; all the seeds are divided into four parts. The seeds of the first part germinated in darkness water for 48 h and then cultured in light for 48 h. The second part of the seeds germinated for 48 h and then transferred to 200 μM CdCl2 solution for stress treatment for 48 h. The third part of the seeds germinated in distilled water for 24 h, then transferred them to petri dish containing 100 μM NaHS to pretreat for 24 h in the dark, and finally transferred seedlings to another petri dish containing 200 μM CdCl2 for stress treatment for 48 h. The fourth part of seeds germinated in distilled water for 36 h and then transferred them to 400 μM hypotaurine (HT, a scavenger of H2S) to pretreat for 12 h in the dark and finally replaced with 200 μM CdCl2 solution for cultivation for 48 h. Light culture was carried out during Cd stress treatment for 48 h, the condition of illuminating incubation climate box was set to 25 ± 1 °C, 12 h photoperiod and photosynthetically active radiation = 200 μmol m−2 s−1. Then, the root length and fresh weight of seedling were measured.
Detection of Cell Death
Evans blue was used for cell death detection. The seedling root tips (2 cm) treated in different treatments were excised and stained with 0.1% (w/v) Evans blue for 15 min and washed them with water for 10 min and then taken pictures with fluorescence microscope (Revolve RVL-100-G, ECHO, USA). Finally, the stained samples were extracted with 1 mL 80% ethanol and bathed in water at 50 °C for 15 min and then centrifuged at 10,000 g for 10 min; the absorbance was measured at 600 nm (Zhang et al. 2015a).
Detection of Endogenous H2S Content and Mitochondrial H2O2 Content
According to the method of determining H2S content by Fang et al. (Fang et al. 2014b), 0.2 g root sample was added with 5 mL 50 mM phosphate buffer solution (0.2 M ascorbic acid (AsA), 0.1 M EDTA, and 0.5 mL 1 M HCl, PH 6.8) was added to homogenate, the released H2S was caught by 1% (w/v) zinc acetate. After 30 min, 0.3 mL 5 mM dimethyl-p-phenylenediamine dissolved in 3.5 mM H2SO4 added to the mixing and then 0.3 mL of 50 mM ferric ammonium sulfate was added. After 15 min of reaction, the value of absorption at 667 nm was detected.
According to the method of He et al. (He et al. 2018), 1 mL of the isolated mitochondrial extract was taken and put into the test tube; 1 mL of 0.1 M phosphoric acid buffer (pH 7.0) and 2 mL KI were added, mixed, and shaken well and allowed to stand for 20 min at room temperature and then the change of absorption value at 390 nm was measured.
Mitochondrial Extraction from Root Tip
Mitochondrion was isolated from root tips of 4-d-old cucumber seedlings using a kit (Bestbio, BB-3611-1, China) and following the manufacture’s instruction. 1 g root sample was added with 3 mL extract to grind on ice. The sample was homogenized at 4 °C, centrifuged at 100 g for 1 min, collected supernatant and discarded sediment. The filtrate was centrifuged at 700 g for 10 min and the supernatant was collected. Supernatant 11,000 g was centrifuged for 20 min and the supernatant was discarded. 1.5 mL of mitochondrial preservation solution was added to the precipitation and resuspended and then suspension at 11,000 g was centrifuged for 15 min, the supernatant was discarded, and the precipitation was mitochondria. The isolated mitochondria were resuspended with suspension and stored in refrigerator at 4 °C for further experiments.
Determination of Mitochondrial Membrane Permeability Transition Pore (MPTP) and Cyt c/a
The method of determination is referred to He et al. (He et al. 2018). The isolated mitochondria were suspended in buffer solution (220 mM mannitol, 70 mM sucrose, 5 mM HEPES, 5 mM sodium succinate, pH 7.2) with a protein concentration of 0.3 mg/mL at 20 °C for 2 min, and then, UV light photometer was used to detect the absorbance value of 540 nm.
The isolated mitochondria were suspended with 0.2% BSA with a protein concentration of 0.5 mg/mL, and then, the absorption values of 550 nm and 630 nm were detected. The absorption values of the two wavelengths were Cyt c/a.
Determination of Ca2+ in Mitochondria
Mitochondrial extract (1.5 mL) added 5 mL concentrated nitric acid and mixed solution was cultured at 25 °C for 7 days, and then, 1% LaCl3 was added to make the volume 10 mL. The absorption value was measured by flame atomic spectrophotometer, and the concentration was calculated according to the Ca2+ standard curve (Li et al. 2018a, 2018b).
Determination of Ca2+-ATPase, H+-ATPase, and Mg2+-ATPase Activity
Ca2+-ATPase, H+-ATPase, and Mg2+-ATPase activity were measured according to Blumwald’s method (Blumwald and Poole 1987). H+-ATPase reaction system includes 30 mmol/L HEPES-Tris, 50 mmol/L KCl, 30 mmol/L MgSO4, 0.1 mmol/L ammonium molybdate, 0.1 mmol/L Na3VO4, and 100 μL membrane proteins and then adds 50 μL 3 mmol/L ATP-Tris for incubation at 37 °C for 30 min. Reaction system was added with 50 μL 55% three chloroacetic acid to terminate the reaction. Finally, the reaction solution was added with 2.5 mL phosphorus-free protectant and 250 μL 6.47 mmol/L NaOH to continue the reaction for 40 min. The absorbance was measured at 550 nm.
Ca2+-ATPase activity is determined according to H+-ATP. In the reaction system, the activity difference caused by adding or not adding 50 μL 3 mmol/L Ca(NO3)2 was taken as the Ca2+-ATPase activity. Similarly, the activity difference caused by adding or not adding 30 mmol/L MgSO4 was taken as the Mg2+-ATPase activity.
The Activity of Caspase-3-Like
Caspase-3-like assay kit (solarbio, BC3830) was used according to the manufacture’s instruction. To measure the activity of caspase-3-like, assays were performed on 96-well microtitre plates by incubating 35 μL extraction solution and 65 μL reaction buffer [5 μL caspase-3 substrate (DEVD-pNA, 2 mM)]. Lysates were incubated at 37 °C for 4 h. The mixture was measured with an ELISA reader (CMax Plus, Molecular Devices, USA) at 405 nm.
Determination of CsVDAC and CsANT Expression by Quantitative RT-PCR
Total RNA was extracted from peanut root tips after different treatments for 24 h with an RNAiso plus kit (TaKaRa Inc., Japan) according to the manufacturer’s instructions. The cDNA was amplified using the following primers shown in Table 1.
Statistical Analysis
All the experiments in this study were repeated three times, and the results shown are the mean ± SE of three independent experiments. Data analysis was used for Duncan’s multiple test (P < 0.05) using SPSS 19.0 software (IBM SPSS, Chicago, USA).
Results
H2S Alleviated the Inhibition of Cd Stress on Root Length and Fresh Weight
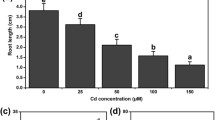
Observations about the effect of different treatments on the root length and fresh weight are shown in Fig. 1A–B. Treatment with 200 μM Cd caused significant decrease of 48.85% and 19.32% in root length and fresh weight, respectively, reference to CK. Pretreatment with HT enhanced Cd toxicity and reduced root length by 51.86% and fresh weight by 22.67%, respectively. However, exogenously supplied NaHS alleviated toxic effect of Cd by increasing the root length and fresh weight by 28.32% and 14.78%, respectively, in comparison to Cd-alone-treated seedlings. In comparison to HT + Cd-treated plants, treatment of NaHS + Cd led to 36.34% and 19.76% increase in root length and fresh weight. These results showed that H2S could alleviate the inhibition of root length and fresh weight under Cd stress.
Effects of H2S on root length and fresh weight of cucumber seedlings under Cd stress. CK: distilled water; Cd: 200 μM; NaHS + Cd: pretreatment with 100 μM NaHS for 24 h and then added 200 μM CdCl2; HT + Cd: pretreatment with 400 μM HT for 12 h and then added 200 μM CdCl2. Determination of root length (A) and fresh weight (B) after 48 h. The results shown are means ± SE (n = 15) of three independent experiments. Different letters indicate statistically significant difference (P < 0.05; Duncan’s multiple test)
H2S Promoted Root Length and Fresh Weight by Reducing Root Tip Cell Death
To investigate whether the inhibition of Cd on root length and fresh weight was caused by the cell death of root tip, we measured degree of cell death by Evans blue. Fig. 2A shows that staining degree of root tips cell death under different treatments, roots treated with Cd and HT showed a deeper staining level compared with CK and NaHS + Cd. Likewise, roots staining extract showed similar phenomena (Fig. 2B). In order to quantify the root tips cell death under different treatments, we measure the OD value of Evans blue staining. The results showed that Cd stress significantly increased cell death, especially HT + Cd. Compared with CK, Cd and HT + Cd treatments increased by 151.14% and 177.12%, respectively. Pretreatment with 100 μM NaHS significantly reduced cell death by 17.47%, 25.21% compared with Cd and HT + Cd, repetitively. These results demonstrated that H2S could alleviate Cd-induced cell death, while the elimination of H2S (HT) aggravated cell death.
Effects of different treatments on cell death in cucumber seedlings root. A Roots stained with Evans blue observed under a light microscope. B Quantitative analysis of root cell death in cucumber seedlings. The results shown are means ± SE of three independent experiments. Different letters indicate statistically significant difference (P < 0.05; Duncan’s multiple test). FW fresh weigh
Change of H2S and Mitochondrial H2O2 Content of Cucumber Seedlings Root Tips at Different Treatments Under Cd Stress
To examine whether H2S and mitochondrial H2O2 are involved in Cd-induced PCD, the effects of Cd and H2S on mitochondrial H2S and H2O2 were investigated. The measurement results are shown in Fig. 3. After 48 h with Cd alone, endogenous H2S and mitochondrial H2O2 content significantly raised by 40.00% and 96.78% compared with CK. Under treatments of H2S scavenger HT, mitochondrial H2O2 content was higher level than other treatments. On the contrary, endogenous H2S content was lower level than other treatments. However, pretreatment with 0.1 mM NaHS not only increased endogenous H2S content by 76.84% and 26.32% (CK and Cd) but also decreased mitochondrial H2O2 content by 22.31% and 36.49% compared with Cd and HT + Cd, respectively. These results indicated that the decrease of endogenous H2S aggravated the oxidation of mitochondrion; NaHS pretreatment could protect mitochondrion from oxidative damage by increasing endogenous H2S level and reducing mitochondrial H2O2 content.
Change of H2S and H2O2 content of cucumber seedlings root tips at different treatments. CK: distilled water; Cd: 200 μM CdCl2; NaHS + Cd: 100 μM NaHS pretreatment for 24 h + Cd; HT + Cd: 400 μM HT pretreatment for 12 h + Cd. H2O2 content in mitochondria of root tip cells and H2S content in roots was determined under Cd stress for 48 h. The results are means ± SE of three independent experiments. Different letters show significant differences (P < 0.05; Duncan’s multiple test). FW fresh weight
Effect of H2S on Mitochondrial Ca2+ Concentration and Mitochondrion Ca2+-ATPase, H+-ATPase, and Mg2+-ATPase Activities Under Cd Stress
To investigate whether increased cytosolic Ca2+ levels in cucumber seedling root tips are due to the release of mitochondrial Ca2+ into cytoplasm, mitochondrial Ca2+ concentration was measured. Cd and HT + Cd significantly decreased mitochondrial Ca2+ levels in root tips (Fig. 4A). NaHS + Cd significantly alleviated the decrease of mitochondrial Ca2+ concentration. Compared with Cd alone, pretreatment with 100 μM NaHS increased by 22.64%. Compared with HT + Cd, NaHS + Cd raised mitochondrial Ca2+ level by 44.73%.
Change of mitochondrion Ca2+ concentration, mitochondrion Ca2+-ATPase activity, H+-ATPase activity, and mitochondrion Mg2+-ATPase activity with different treatments under Cd stress. CK: distilled water; Cd: 200 μM CdCl2; NaHS + Cd: 100 μM NaHS pretreatment for 24 h + Cd; HT + Cd: 400 μM HT pretreatment for 12 h + Cd. After 48 h, mitochondrion Ca2+ concentration (A), mitochondrion Ca2+-ATPase activity (B), H+-ATPase activity (C), and mitochondrion Mg2+-ATPase activity (D) were determined. The results are means ± SE of three independent experiments. Different letters show significant differences (P < 0.05; Duncan’s multiple test)
To investigate whether H2S could maintain mitochondrial chemical potential and stabilize membrane structure, we measured mitochondrion Ca2+-ATPase activity, H+-ATP activity, and mitochondrion Mg2+-ATP activity. Under Cd stress, both mitochondrion Ca2+-ATPase activity (Fig. 4B), H+-ATPase activity (Fig. 4C), and Mg2+-ATPase activity (Fig. 4D) decreased significantly, especially under HT + Cd. Compared with Cd alone, pretreatment with NaHS increases mitochondrion Ca2+-ATPase activity (32.12%), H+-ATPase activity (24.72%), and mitochondrion Mg2+-ATPase activity (18.25%). These data suggested that H2S could inhibit the destruction of mitochondrial chemical potential and membrane stability by Cd stress and reduce the efflux of mitochondrial Ca2+.
Effect of H2S on Mitochondrial Cyt c/a, MPTP, and Caspase-3-Like Activity Under Cd Stress
As shown in Fig. 5A–B, Cd stress markedly decreased Cyt c content and the OD value of MPTP by 28.42% and 37.17% compared with CK. Moreover, after application of H2S scavenger HT, Cyt c/a and MPTP showed lower than CK and Cd alone. Under NaHS pretreatment, Cyt c/a and MPTP were 25.20% and 34.90% higher than Cd stress. Likewise, pretreatment with NaHS increased by 48.16% and 67.93% compared with HT + Cd. We measured caspase-3-like activity (Fig. 5C). The results showed that caspase-3-like activity of Cd stress and HT + Cd were 2.10 and 2.76 times higher than CK, respectively. Pretreatment with 0.1 mM NaHS significantly decreased caspase-3-like activity compared with Cd and HT + Cd treatments. The above results suggested that H2S inhibited the opening of MPTP, release of mitochondrial Cyt c and reduced caspase-3-like activity under Cd stress.
Effects of different treatments on mitochondrion Cyt c/a, MPTP, and caspase-3-like activity under Cd stress. 2-day-old cucumber seedlings were treated by different treatments for 48 h and then mitochondrion Cyt c/a (A), MPTP (B), and caspase-3-like (C) activity were determined. The results are means ± SE of three independent experiments. Different letters show significant differences (P < 0.05; Duncan’s multiple test)
H2S Downregulates Expression of MPTP-Related Genes to Prevent Loss of Ca2+
Furthermore, CsVDAC2, CsVDAC4, CsANT1, and CsANT2 gene expression was as a molecular probe to investigate the molecular mechanism of H2S regulating MPTP. Compared with CK, Cd and HT + Cd treatments were able to induce high expression of CsVDAC2, CsVDAC4, CsANT1, and CsANT2. These gene expressions were matched with the opening of MPTP. It was worth noting that NaHS pretreatment down-regulated these gene expressions by 24.63%, 32.15%, 27.87%, and 21.65%, respectively. These results showed that H2S down-regulated CsVDAC and CsANT expression to inhibit the opening of MPTP.
Discussion
H2S is a signal molecule involved in regulating physiological processes of animals and plants (García‐Mata and Lamattina 2010; Li et al. 2011). In addition, H2S is involved in heavy metal stress (Ahmad et al. 2019; Mostofa et al. 2015; Qian et al. 2014). Our study showed that Cd treatment significantly inhabited root elongation and fresh weight of cucumber seedlings (Fig. 1). Moreover, Cd stress led to root tips cell death, while pretreatment with 0.1 mM NaHS increased root length and fresh weigh by reducing cell death (Figs. 1, 2). These findings were consistent with those reported in the literature. Zhang et al. reported that 5 mM CdCl2 seriously affected the root growth of cabbage seedlings and markedly caused root tips cell death. When seedlings were treated with 5 μM for 24 h, the inhibition effect of Cd stress was significantly reduced (Zhang et al. 2015a). In rice seedlings, 0.5 mM CdCl2 significantly decreased plant height, fresh weight, and dry weight; Evans blue staining showed that Cd stress caused cell death. NaHS treatment (0.1 mM) could alleviate Cd toxicity to maintain growth (Mostofa et al. 2015). Thus, all findings presented here indicate that NaHS appears to positively effect in plant growth.
The accumulation of endogenous H2S is the response of plants to stress. In this study, Cd stress led to the accumulation of endogenous H2S content that was higher than CK. Pretreatment with NaHS strengthened this accumulation effect (Fig. 3). High-concentration heavy metal ions can stimulate a ROS burst (Dai et al. 2016; Qian et al. 2014; Zhang et al. 2015a), which is one of the inducers of cell death (Jabs 1999). Figure 3 shows that mitochondria H2O2 accumulation was stimulated by Cd stress. NaHS + Cd treatment significantly decreased this accumulation, which was consistent with other reports (Huang et al. 2016). Mitochondria are generally considered to be an important site of ROS-mediated PCD events; the release of Cyt c and collapse of MPTP are also reported to be one of the vital events in PCD (He et al. 2018; Yao et al. 2004). Mitochondria are involved in cell apoptosis of plants (Rurek 2014); because of the increased mitochondrial membrane permeability and decreased membrane potential, mitochondrial Ca2+ and Cyt c were released into the cytoplasm, causing an increase in cytosolic Ca2+ levels. We also confirmed that Cd induced the release of mitochondria Ca2+ (Fig. 4A) and decreased mitochondria Cyt c/a (Fig. 5A). Li et al. also found that allelochemicals induced cell death in maize root tip, which was released from mitochondria with Cyt c and Ca2+ (Li et al. 2018a, 2018b). Cyt c release has been seen also in plant cells under-going opening of MPTP and subsequent PCD under the influence of biotic and abiotic stress conditions (Panda et al. 2008). We noticed that the OD value of MPTP also decreased by Cd stress (Fig. 5B). This is consistent with He et al. who reported that aluminum caused Cyt c/a and OD value of MPTP in peanut root (He et al. 2018). In our study, Cd stress induced up-regulation of VDAC and ANT expression that indicated the opening of MPTP. NaHS pretreatment significantly down-regulated VDAC and ANT expression to inhibit the opening of MPTP compared with Cd, while scavenger HT treatment exacerbates this condition (Fig. 6). Similarly, in a rat model of ischemia-reperfusion, H2S prevents cardiomyocyte apoptosis by inhibiting the opening of MPTP (Yao et al. 2010; Zhang et al. 2013). These results suggested that H2S as an antioxidant protected mitochondrial function from Cd toxicity.
Effects of different treatments on the expression of CsVDC2, CsVDAC4, CsANT1, and CsANT2. After 24 h treatment, the relative expressions of CsVDC2 (A), CsVDAC4 (B), CsANT1 (C), and CsANT2 (D) were detected by real-time fluorescence quantitative analysis under different treatments. The results are means ± SE of three independent experiments. Different letters show significant differences (P < 0.05; Duncan’s multiple test)
ATP is the energy to maintain plant life activities, and H+-ATPase, Ca2+-ATPase, and Mg2+-ATPase located on plasma membrane is an important ATPase. They play a key role in maintaining the chemical potential inside and outside the membrane and stabilizing the function of plasma membrane. Ca2+-ATPase is an important regulator of Ca2+ transport in plants, which can maintain the gradient of Ca2+ concentration intracellular and extracellular (Ferrol and Bennett 1996). Similar studies are reported (Ahn et al. 2001; Zhan et al. 2014; Zhao et al. 2008). Our study demonstrated that Cd stress decreased H+-ATPase, Ca2+-ATPase, and Mg2+-ATPase activity (Fig. 4B–D), but pretreatment with NaHS maintained mitochondrial ATPase activity and inhibited mitochondrial Ca2+ efflux into cytoplasm (Fig. 4A). When Ca2+-ATPase was activated, mitochondrial Ca2+ exchanged with cytoplasmic Ca2+ to maintain balance. Cd stress decreased Ca2+-ATPase enzyme activity and increased mitochondrial membrane permeability, breaking this balance and led to [Ca2+]cyt overload. Dawood and Li have similar results to ours (Dawood et al. 2012; Juan et al. 2018). These results showed that H2S could maintain mitochondria ATP activity to limit mitochondria Ca2+ efflux and [Ca2+]cyt overload.
Cd can cause plant chlorosis, growth inhibition, and cell death due to its toxicity; the typical PCD upon Cd treatment were observed in cell suspensions of tobacco (Kuthanova et al. 2008; Ma et al. 2010), tomato (Elena et al. 2005; Gallego et al. 2012), and Arabidopsis (De Michele et al. 2009). Our results confirmed that Cd induced the occurrence of cell death caspase-3-like enzyme activation (Fig. 5C). Caspase activity plays an important role in the process of PCD and caspase-3 is the executor of PCD (Li and Xing 2010). Cd treatment induced the increase of caspase-3-like activity significantly (Fig. 5C). Exogenous NaHS pretreatment reduced the occurrence of PCD, while HT (H2S scavenger) could accelerate the process of cell death.
Conclusion
Taken together, Cd-induced cell death in cucumber root tips was mitochondria-dependent cell death. Cd stress damaged mitochondrial physiological functions by mitochondrial H2O2 accumulation and the opening of MPTP to release Cyt c and Ca2+. H2S prevented cell death of Cd-induced by improving mitochondrial physiological properties and a potential schematic diagram is shown in Fig. 7.
Schematic diagram for the effect of H2S on cell death induced by Cd. The Figure shows that H2S/H2O2 may mediate Cd-induced PCD via mitochondria-dependent pathway as a mixed signal. The yellow and blue circles represent Cyt c and Ca2+, respectively. HT hypotaurine, a scavenger of H2S; MPTP mitochondrial permeability transition pore; VDAC voltage-dependent anion channel; ANT adenine nucleotide translocator
Abbreviations
- H2S:
-
Hydrogen sulfide
- PCD:
-
Programmed cell death
- CdCl2 :
-
Cadmium chloride
- Cyt c/a:
-
Cytochrome c/a
- MPTP:
-
Mitochondrial membrane permeability transition pores
- ATPase:
-
Adenosine triphosphatase
- NaHS:
-
Sodium hydrosulfide
- HT:
-
Hypotaurine
- H2O2 :
-
Hydrogen peroxide
- VDAC:
-
Voltage-dependent anion channel
- ANT:
-
Adenine nucleotide translocator
References
Ahmad R, Ali S, Rizwan M, Dawood M, Ahmad P (2019) Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plant 168:289–300
Ahn SJ, Sivaguru M, Osawa H, Chung GC, Matsumoto H (2001) Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potentials in squash roots. Plant Physiol 126:1381–1390
Anja R, Kerstin R, Rainer H, Jutta P, Holger H (2005) Impact of reduced O-acetylserine(thiol)lyase isoform contents on potato plant metabolism. Plant Physiol 137:892–900
Bernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79:1127–1155
Berridge MJ, Bootman MD, Llewelyn H (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529
Blumwald E, Poole RJ (1987) Salt tolerance in suspension cultures of sugar beet: induction of Na+/H+ antiport activity at the tonoplast by growth in salt. Plant Physiol 83:884–887
Boursiac Y, Harper JF (2007) The origin and function of calmodulin regulated Ca2+ pumps in plants. J Bioenerg Biomembr 39:409–414
Chen S, Wang X, Jia H et al (2021) Persulfidation-induced structural change in SnRK2. 6 establishes intramolecular interaction between phosphorylation and persulfidation. Mol Plant. https://doi.org/10.1016/j.molp.2021.07.002
Cheng W, Zhang L, Jiao C, Su M, Yang T, Zhou L, Peng R, Wang R, Wang C (2013) Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol Biochem 70:278–286
Crompton M, Barksby E, Johnson N, Capano M (2002) Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie 84:143–152
Dai H, Xu Y, Zhao L, Shan C (2016) Alleviation of copper toxicity on chloroplast antioxidant capacity and photosystem II photochemistry of wheat by hydrogen sulfide. Braz J Bot 39:787–793
Dawood M, Cao F, Jahangir MM, Zhang G, Wu F (2012) Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J Hazard Mater 209:121–128
De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M, Careri M, Zottini M, di Toppi LS, Schiavo FL (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150:217–228
Elena I, Veneta KT, Anke DJ, Atanas A (2005) Involvement of ethylene, oxidative stress and lipid-derived signals in cadmium-induced programmed cell death in tomato suspension cells. BioMed Central 5:1–2
Fang H, Jing T, Liu Z, Zhang L, Jin Z, Pei Y (2014a) Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 56:472–481
Fang T, Cao Z, Li J, Shen W, Huang L (2014b) Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem 76:44–51
Fang H, Liu Z, Long Y, Liang Y, Jin Z, Zhang L, Liu D, Li H, Zhai J, Pei Y (2017) The Ca2+/CaM2 binding transcription factor TGA3 elevates LCD expression and H2S production to bolster Cr6+ tolerance in Arabidopsis. Plant J 91:1038–1050
Ferrol N, Bennett AB (1996) A single gene may encode differentially localized Ca2+-ATPases in tomato. Plant Cell 8:1159–1169
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
García-Mata C, Lamattina L (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188:977–984
Giorgi C, Baldassari F, Bononi A, Bonora M, Marchi ED, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM (2012) Mitochondrial Ca2+ and apoptosis. Cell Calcium 52:36–43
Guan MY, Zhang HH, Pan W, Jin CW, Lin XY (2018) Sulfide alleviates cadmium toxicity in Arabidopsis plants by altering the chemical form and the subcellular distribution of cadmium. Sci Total Environ 627:663–670
Hancock JT, Whiteman M (2014) Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol Biochem 78:37–42
Harada A, Sakai T, Okada K (2003) Phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100:8583–8588
He H, Huang W, Oo TL, Gu M, Zhan J, Wang A, He LF (2018) Nitric oxide suppresses aluminum-induced programmed cell death in peanut (Arachis hypoganea L.) root tips by improving mitochondrial physiological properties. Nitric Oxide 74:47–55
Huang H, Shah K, Bradbury N, Li C, White C (2014) Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis 5:e1482–e1482
Huang Z-Q, Ye S-C, Hu L-Y, Hu K-D, Yan H, Li W-J, Jiao H, Zhang H (2016) Hydrogen sulfide promotes wheat grain germination under cadmium stress. Proc Natl Acad Sci, India Sect B: Biol Sci 86:887–895
Jabs T (1999) Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol 57:231–245
Javadov SA, Lim KHH, Kerr PM, Suleiman MS, Angelini GD, Halestrap AP (2000) Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res 45:360–369
Jia H, Chen S, Liu D et al (2018) Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front Plant Sci 9:1517
Jones A (2002) Programmed cell death during tracheary element differentiation. Phytopathology 92 (6 supplement).
Jörg K, Oliver B, Kenji H (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22:541–563
Juan LI, Tian BH, Liu DM, Pei YX (2018) Hydrogen sulfide regulates cadmium stress resistance through P-ATPases in foxtail millet seedlings. J Agro-Environ Sci 37:52–57
Kou N, Xiang Z, Cui W, Li L, Shen W (2018) Hydrogen sulfideacts downstream of methane to inducecucumber adventitious root development. J Plant Physiol 228:113–120
Kusano T, Tateda C, Berberich T, Takahashi Y (2009) Voltage-dependent anion channels: their roles in plant defense and cell death. Plant Cell Rep 28:1301–1308
Kuthanova A, Fischer L, Nick P, Opatrny Z (2008) Cell cycle phase-specific death response of tobacco BY-2 cell line to cadmium treatment. Plant Cell Environ 31:1634–1643
Leng FW (2012) On Ca signalling research. Sci China Life Sci 55:744–746
Li Z, Xing D (2010) Mechanistic study of mitochondria-dependent programmed cell death induced by aluminium phytotoxicity using fluorescence techniques. J Exp Bot 62:331–343
Li L, Rose P, Moore PK (2011) Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187
Li Z-G, Gong M, Xie H, Yang L, Li J (2012) Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci 185:185–189
Li ZG, Long WB, Yang SZ, Wang YC, Tang JH, Wen L, Zhu BY, Min X (2015) Endogenous hydrogen sulfide regulated by calcium is involved in thermotolerance in tobacco Nicotiana tabacum L. suspension cell cultures. Acta Physiol Plant 37:1–11
Li J, He Y, Ma D, Bing H, Wang Y, Chen B (2018a) Volatile allelochemicals of Chenopodium ambrosioides L. induced mitochondrion-mediated Ca2+-dependent and caspase-dependent apoptosis signaling pathways in receptor plant cells. Plant Soil 425:1–12
Li J, Chen S, Wang X et al (2018b) Hydrogen sulfide disturbs actin polymerization via S-sulfhydration resulting in stunted root hair growth. Plant Physiol 178(2):936–949
Lin J, Wang Y, Wang G (2005) Salt stress-induced programmed cell death via Ca2+-mediated mitochondrial permeability transition in tobacco protoplasts. Plant Growth Regul 45:243–250
Lin J, Wang Y, Wang G (2006) Salt stress-induced programmed cell death in tobacco protoplasts is mediated by reactive oxygen species and mitochondrial permeability transition pore status. J Plant Physiol 163:731–739
Liu J, Niu Y, Zhang J, Zhou Y, Zheng M, Xuan H (2018) Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: recent advances. Plant Cell, Tissue Organ Cult 132:413–424
Ma W, Xu W, Xu H, Chen Y, He Z, Ma M (2010) Nitric oxide modulates cadmium influx during cadmium-induced programmed cell death in tobacco BY-2 cells. Planta 232:325–335
Ma Y, Zhang W, Niu J, Ren Y, Zhang F (2019) Hydrogen sulfide may function downstream of hydrogen peroxide in salt stress-induced stomatal closure in Vicia faba. Funct Plant Biol 46:136–145
Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P (2018) Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 69:62–72
Mostofa MG, Rahman A, Ansary MMU, Watanabe A, Fujita M, Tran L-SP (2015) Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep 5:1–17
Panda SK, Yamamoto Y, Kondo H, Matsumoto H (2008) Mitochondrial alterations related to programmed cell death in tobacco cells under aluminium stress. CR Biol 331:597–610
Qian P, Sun R, Basharat B, Ali B, Gill RA, Xu L, Zhou W (2014) Effects of hydrogen sulfide on growth, antioxidative capacity, and ultrastructural changes in oilseed rape seedlings under aluminum toxicity. J Plant Growth Regul 33:566–578
Riemenschneider A, Wegele R, Schmidt A, Papenbrock J (2010) Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J 272:1291–1304
Rimessi A, Giorgi C, Pinton P, Rizzuto R (2008) The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta 1777:808–816
Rosario R, Diego DS, Anna R, Cristina M (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13:566–578
Rurek M (2014) Plant mitochondria under a variety of temperature stress conditions. Mitochondrion 19:289–294
Sabine F, Yong-Fei W, Chris S, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci U S A 104:14531–14536
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14:S401–S417
Shintaro M, Kenji O, Megumi W-S, Yoshimasa N, Yasuaki S, Yoshiyuki M (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol 143:1398–1407
Sisalli MJ, Savoia C, Scorziello A (2012) Mitochondrial Ca2+ dysregulation during stroke and cell death. Metal Ion in Stroke. Springer 2012: 41-67
Xie Y, Zhang C, Lai D, Sun Y, Samma MK, Zhang J, Shen W (2014) Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J Plant Physiol 171:53–62
Yao N, Eisfelder BJ, Marvin J, Greenberg JT (2004) The mitochondrion - an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J 40:596–610
Yao L-L, Huang X-W, Wang Y-G, Cao Y-X, Zhang C-C, Zhu Y-C (2010) Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3β-dependent opening of mPTP. Am J Physiol Heart Circul Physiol 298:H1310–H1319
Yuan S, Fu Y, Wang X, Shi H, Huang Y, Song X, Li L, Song N, Luo Y (2008) Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis. FASEB J 22:2809–2820
Zhan J, Li W, He H-y, Li C-z, He L-f (2014) Mitochondrial alterations during Al-induced PCD in peanut root tips. Plant Physiol Biochem 75:105–113
Zhang Q, Fu H, Zhang H, Xu F, Zou Z, Liu M, Wang Q, Miao M, Shi X (2013) Hydrogen sulfide preconditioning protects rat liver against ischemia/reperfusion injury by activating Akt-GSK-3β signaling and inhibiting mitochondrial permeability transition. PLoS ONE 8:e74422
Zhang L, Pei Y, Wang H, Jin Z, Liu Z, Qiao Z, Fang H, Zhang Y (2015a) Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. pekinensis. Oxid Med Cell Longev 2015:1–11
Zhang Y-X, Hu K-D, Lv K, Li Y-H, Hu L-Y, Zhang X-Q, Ruan L, Liu Y-S, Zhang H (2015b) The hydrogen sulfide donor NaHS delays programmed cell death in barley aleurone layers by acting as an antioxidant. Oxid Med Cell Longev. https://doi.org/10.1155/2015/714756
ZHAO S-C, SUN J-W, WANG X-B, WANG H, LIANG G-Q, ZHOU W (2008) Effects of cadmium on calmodulin content and Ca~(2+)-ATPase activities of maize (Zea mays) seedling. Plant Nutr Fertil Sci 2
Zhi Q, Rajeev V, Chris G, Yube Y, Yichen Z, Ryan CA, Berkowitz GA (2010) Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA 107:21193–21198
Acknowledgements
This work was supported by the Industrial support plan project of Gansu Provincial Department of Education (2021CYZC-45), the China Agriculture Research System (CARS-23-B03), the National key research and development projects (2018YFD0201205), the National Natural Science Foundation of China (No. 31660584), China Agriculture Research System (CARS-23-C-07), Gansu Province Science and Technology Key Project Fund (No. 17ZD2NA015), and Natural Science Foundation of Gansu References Province, China (1610RJZA098).
Funding
This study was funded by the Industrial support plan project of Gansu Provincial Department of Education (2021CYZC-45), the China Agriculture Research System (CARS-23-B03), the National key research and development projects (2018YFD0201205), the National Natural Science Foundation of China (No. 31660584), China Agriculture Research System (CARS-23-C-07), Gansu Province Science and Technology Key Project Fund (No.17ZD2NA015), and Natural Science Foundation of Gansu References Province, China (1610RJZA098).
Author information
Authors and Affiliations
Contributions
Conceptualization: SL, ZT, and WL; formal analysis: SL, ZT, and JL; funding acquisition: JY; investigation: SL; methodology: SL, WL, and AC; project administration: GZ and JY; resources: JY; supervision: WL, GZ, and JY; validation: JY; writing—original draft: SL; writing—review and editing: AC and ZL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Vijay Pratap Singh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, S., Tang, Z., Yu, J. et al. Hydrogen Sulfide Inhibits Cadmium-Induced Cell Death of Cucumber Seedling Root Tips by Protecting Mitochondrial Physiological Function. J Plant Growth Regul 41, 3421–3432 (2022). https://doi.org/10.1007/s00344-021-10524-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10524-x