Abstract
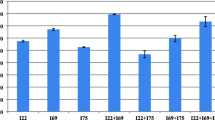
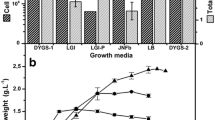
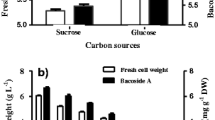
Gibberellic acid (GAs), a vital phytohormone, is necessary to increase seed germination as well as plant’s health and growth. Likewise, a number of plant growth-promoting bacteria produce identical secondary metabolites which serve as plant growth stimulants. The current study focuses to investigate optimization of multiple variables for the maximum GAs production by bacterial isolates via Response surface methodology (RSM) with the help of Box–Behnken design (BBD) and its effects on seed germination and growth promotion of Cicer arietinum (Chickpea). Initially, bacterial isolates were screened on the basis of quantitative production of gibberellic acid without amendment of any precursor. Later, bacterial isolate, MEN8, was selected for peak production of gibberellic acid via BBD and a total of 50 sets of trials were finalized. RSM analysis signified maximum GAs production by the isolate up to 109.25 μg/ml on 5th day of incubation at 35 °C on pH 7.0 by consuming 3 g/l fructose and 1.0 g/l ammonium chloride. The extracted gibberellic acid was purified by using Thin-layer chromatography, elucidated on Rf value 0.72 with gray-colored spot which was further confirmed by High-performance liquid chromatography technique, at 2.68 retention time (Rt). The MEN8 isolate was molecularly identified up to species level as Bacillus cereus by using 16S rRNA gene sequencing and phylogeny. As a final point, in vitro analysis confirmed that B. cereus MEN8 was a significant isolate for increasing seed germination parameters such as germination energy (GE-27%), capacity (GC-32%), index (GI-42%), percentage (GP-55%), vigor index (VI-89%), and vegetative growth parameters including root/shoot length and fresh/dry weight. Plant growth-promoting nature of B. cereus MEN8 creates a future avenue to be utilized as ‘phytohormone-producing bioinoculant’ for sustainable agriculture at commercial implications.





Similar content being viewed by others
References
Aeron A, Dubey RC, Maheshwari DK, Pandey P, Bajpai VK, Kang SC (2011) Multifarious activity of bioformulated Pseudomonas fluorescens PS1 and biocontrol of Sclerotinia sclerotiorum in Indian rapeseed (Brassica campestris L.). Eur J Plant Pathol 131(1):81–93
Afzal I, Shinwari ZK, Sikandar S, Shahzad S (2019) Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol Res 221:36–49
Ambawade MS, Pathade GR (2015) Production of gibberellic acid by Bacillus siamensis BE 76 isolated from banana plant (Musa spp.). Int J Sci Res 4(7):394–398
Arora NK, Mishra J (2016) Prospecting the roles of metabolites and additives in future bioformulations for sustainable agriculture. Appl Soil Ecol 107:405–407
Aydar Y (2018) Utilization of response surface methodology in optimization of extraction of plant materials. In Statistical approaches with emphasis on design of experiments applied to chemical processes, 157–169.
Baliyan N, Dheeman S, Maheshwari DK, Dubey RC, Vishnoi VK (2018) Rhizobacteria isolated under field first strategy improved chickpea growth and productivity. Environ Sustain 4:461–469
Baliyan N, Dhiman S, Dheeman S, Kumar S, Maheshwari DK (2021) Optimization of indole-3-acetic acid using response surface methodology and its effect on vegetative growth of chickpea. Rhizosphere 17:100321
Berrios J, Illanes A, Aroca G (2004) Spectrophotometric method for determining gibberellic acid in fermentation broths. Biotechnol Letter 26:67–70
Bhagyawant SS, Bhadkaria A, Gupta N, Srivastava N (2018) Impact of phytic acid on nutrient bioaccessibility and antioxidant properties of chickpea genotypes. J Food Biochem 42(6):72–78
Bhalla K, Singh SB, Agarwal R (2010) Quantitative determination of gibberellins by high performance liquid chromatography from various gibberellins producing Fusarium strains. Environ Monit Assess 167(4):515–520
Buono MA, Erickson L (1985) Rapid measurement of Candida utilis dry weight with microwave drying. J Food Prot 48(11):958–960
Camara MC, Vandenberghe LP, Rodrigues C, de Oliveira J, Faulds C, Bertrand E, Soccol CR (2018) Current advances in gibberellic acid (GA3) production, patented technologies and potential applications. Planta 248(5):1049–1062
Candau R, Avalos J, Cerda-Olmedo E (1992) Regulation of gibberellin biosynthesis in Gibberella fujikuroi. Plant Physiol 100(3):1184–1188
Desai SA (2017) Isolation and characterization of gibberellic acid (GA3) producing rhizobacteria from sugarcane roots. Biosci Discov 8(3):488–494
Dheeman S, Maheshwari DK, Baliyan N (2017) Bacterial endophytes for ecological intensification of agriculture. Endophytes: biology and biotechnology. Springer, Cham, pp 193–231
Dheeman S, Baliyan N, Dubey RC, Maheshwari DK, Kumar S, Chen L (2019) Combined effects of rhizo-competitive rhizosphere and non-rhizosphere Bacillus in plant growth promotion and yield improvement of Eleusine coracana (Ragi). Can J Microbiol 999:1–14
Dhiman S, Baliyan N, Maheshwari DK (2020) Buffalo dung-inhabiting bacteria enhance the nutrient enrichment of soil and proximate contents of Foeniculum vulgare Mill. Arch Microbiol 202(9):2461–2470
Dong B, Deng Y, Wang H, Gao R, Stephen GUK, Chen S, Chen F (2017) Gibberellic acid signaling is required to induce flowering of chrysanthemums grown under both short and long days. Int J Mol Sci 18(6):1259
Escamilla A, Sanvicente M, Sosa M, Galindo-Leal C (2000) Habitat mosaic, wildlife availability, and hunting in the tropical forest of Calakmul, Mexico. Conserv Biol 14(6):1592–1601
Feller G (2010) Protein stability and enzyme activity at extreme biological temperatures. J Phys Condens Matter 22(32):323–359
Hakim MA, Juraimi AS, Begum M, Hanafi MM, Ismail MR, Selamat A (2010) Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L.). Afr J Biotechnol 9(13):1911–1918
Holbrook AA, Edge WJW, Bailey F (1961) Spectrophotometric method for determination of gibberellic acid. Adv Chem 28(18):159–167
Kader MA (2005) A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J Proc Royal Soc New South Wale 138(1):65–75
Kahlon SS, Malhotra S (1986) Production of gibberellic acid by fungal mycelium immobilized in sodium alginate. Enzyme Microb Technol 8(10):613–616
Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M (2002) The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol 128(4):1264–1270
Kang SM, Khan AL, You YH, Kim JG, Kamran M, Lee IJ (2014) Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J Microbiol Biotechnol 24(1):106–112
Kapoor R, Soni R, Kaur M (2016) Gibberellins production by fluorescent Pseudomonas isolated from Rhizospheric soil of Malus and Pyrus. Int J Agric Environ Biotechnol 9(2):93–199
Karakoc S, Aksoz N (2006) Some optimal cultural parameters for gibberellic acid biosynthesis by Pseudomonas sp. Tur J Bio 30(1):81–85
Kaymakanova M (2009) Effect of salinity on germination and seed physiology in bean (Phaseolus vulgaris L.). Biotechnol Biotechnologic Equ 23(1):326–329
Kępczyński J, Cembrowska-Lech D, Sznigir P (2017) Interplay between nitric oxide, ethylene, and gibberellic acid regulating the release of Amaranthus retroflexus seed dormancy. Acta Physiol Plant 39(12):1–13
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) Mega X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Lale G, Jogdand VV, Gadre RV (2006) Morphological mutants of Gibberella fujikuroi for enhanced production of gibberellic acid. J Appl Microbiol 100(1):65–72
Lanka S, Latha JNL (2015) Response surface methodology as a statistical tool for fermentation media optimization in lipase production by palm oil mill effluent (POME) isolate Emericella nidulans NFCCI 3643. Methodology 4(4):2535–2545
Lebrazi S, Fadil M, Chraibi M, Fikri-Benbrahim K (2020) Screening and optimization of indole-3-acetic acid production by Rhizobium sp. strain using response surface methodology. J Gen Eng Biotechnol 18(1):1–10
Maheshwari DK, Dheeman S, Agarwal M (2015) Phytohormone-producing PGPR for sustainable agriculture. Bacterial metabolites in sustainable agroecosystem. Springer, Cham, pp 159–182
Miceli A, Moncada A, Sabatino L, Vetrano F (2019) Effect of gibberellic acid on growth, yield, and quality of leaf lettuce and rocket grown in a floating system. Agron 9(7):382
Nagai K, Mori Y, Ishikawa S, Furuta T, Gamuyao R, Niimi Y, Ashikari M (2020) Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature 584(7819):109–114
Paleg LG (1965) Physiological effects of gibberellins. Annu Rev Plant Physiol 16(1):291–322
Panigrahi S, Dash D, Rath CC (2018) Characterization of endophytic bacteria with plant growth promoting activities isolated from six medicinal plants. J Exp Biol Agric Sci 6(5):782–791
Rangaswamy V, Guduri B (2010) Process for gibberellic acid production with Fusarium moniliforme strains. U.S. Patent 7:846–699
Rao PP, Birthal PS, Bhagavatula S, Bantilan MCS (2010) Chickpea and pigeonpea economies in Asia: facts, trends and outlook.
Salazar-Cerezo S, Martínez-Montiel N, Garcia-Sanchez J, Perez-y-Terron R, Martinez-Contreras RD (2018) Gibberellin biosynthesis and metabolism: a convergent route for plants, fungi and bacteria. Microbiol Res 208:85–98
Sarkar S, Sarkar A (2019) Role of irrigation and mulch in chickpea (Cicer arietinum L.) growth, productivity and moisture extraction pattern in Alluvial Zone of West Bengal, India. Legume Res Int J 42(1):77–83
Sasirekha B, Shivakumar S (2012) Statistical optimisation for improved indole-3-acetic acid (IAA) production by Pseudomonas aeruginosa and demonstration of enhanced plant growth promotion. J Soil Sci Plant Nutr 12(4):863–873
Shahzad R, Waqas M, Khan AL, Asaf S, Khan MA, Kang SM, Lee IJ (2016) Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol Biochem 106:236–243
Shamsi IH, Sagonda T, Zhang X, Zvobgo G, Joan HI (2019) The role of growth regulators in senescence. In senescence signalling and control in plants, Academic Press, 99–110.
Sharma S, Sharma A, Kaur M (2018) Extraction and evaluation of gibberellic acid from Pseudomonas spp. plant growth promoting rhizobacteria. J Pharma Phytochem 7(1):2790–2795
Siddikee MA, Hamayun M, Han GH, Sa TM (2010) Optimisation of gibberellic acid production by Methylobacterium oryzae CBMB20. Kor J Soil Sci Fert 43(4):522–527
Silpa D, Rao PB, Kumar GK, Raghu RM (2018) Studies on gibberellic acid production by Bacillus Licheniformis DS3 isolated from banana field soils. Int J Sci Res Sci Technol 4(5):1106–1112
Tahir HA, Gu Q, Wu H, Raza W, Hanif A, Wu L, Gao X (2017) Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front Microbiol 8:171
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tikle A, Mishra A (2018) Physical and milling properties of chickpea, Cicer arietinum influenced by seed characteristics. Biosci Biotechnol Res Comm 11(1):122–127
Tsukanova KA, Meyer JJM, Bibikova TN (2017) Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. South Afr J Bot 113:91–102
Tudzynski B (2005) Gibberellin biosynthesis in fungi: genes, enzymes, evolution, and impact on biotechnology. Appl Microbiol Biotechnol 66(6):597–611
Vashisth A, Nagarajan S (2008) Exposure of seeds to static magnetic field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.). Bioelectrom 29(7):571–578
Werle LB, Abaide ER, Felin TH, Kuhn KR, Tres MV, Zabot GL, Mazutti MA (2020) Gibberellic acid production from Gibberella fujikuroi using agro-industrial residues. Biocatal Agric Biotechnol 25:101608
Acknowledgements
The authors wish to thank the Head, Department of Botany and Microbiology, Gurukul Kangri Deemed to be University, Haridwar (India) for providing necessary facilities.
Author information
Authors and Affiliations
Contributions
NB designed and conceived the experiment with the help of SVD. SD, SK, and PK helped to prepare the manuscript. NKA and DKM corrected and finalized the manuscript before final submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Thomas Roitsch.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baliyan, N., Dhiman, S., Dheeman, S. et al. Optimization of Gibberellic Acid Production in Endophytic Bacillus cereus Using Response Surface Methodology and Its Use as Plant Growth Regulator in Chickpea. J Plant Growth Regul 41, 3019–3029 (2022). https://doi.org/10.1007/s00344-021-10492-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10492-2