Abstract
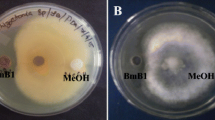
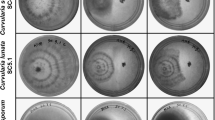
Blossom blight caused by Botrytis cinerea is one among the most devastating diseases that cause complete post-harvest loss in flower crops. The present study focuses on the development of effective bioformulation towards suppression of blossom blight and plant growth promotion in rose. Bacillus amyloliquefaciens (VB2) and Bacillus subtilis (AP) effectively inhibited mycelial growth of B. cinerea in vitro. Genome screening of VB2 and AP revealed the presence of antimicrobial peptide genes including, ituD, ipa14, bacA, bacD, srfA, sfP, spaC, spaS responsible for the biosynthesis of antibiotics such as iturin, bacilysin, bacillomycin, surfactin and subtilin. Further, the presence of volatile antifungal compounds in the bacterial secretome was identified through gas chromatography–mass spectrometry (GC/MS) analysis. Upon treatment, AP accelerated the metabolite profile of the plants and a rise in peak area of antifungal compounds such as, pentadecanoic acid, n-hexadecanoic acid, octadecanoic acid (stearic acid) and tetradecanoic acid was observed. In vitro, VB2 produced maximum indole acetic acid (9.17 µg/ml) and gibberellic acid (8.20 µg/ml) in nutrient broth. Under field conditions, foliar spray of VB2 at 0.5% (5 ml/l), four times at weekly interval suppressed blossom blight incidence (64% reduction over control) and also promoted yield. Future research towards development of an effective bioformulation with extended shelf life will aid in the management of various fungal, bacterial and viral diseases in different crop plants.








Similar content being viewed by others
References
AbuQamar SF, Moustafa K, Tran LS (2016) ‘Omics’ and plant responses to Botrytis cinerea. Front Plant Sci 7:1658
AbuQamar S, Moustafa K, Tran LS (2017) Mechanisms and strategies of plant defense against Botrytis cinerea. Crit Rev Biotechnol 37(2):262–274
Agoramoorthy G, Chandrasekaran M, Venkatesalu V, Hsu M (2007) Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz J Microbiol 38(4):739–742
Asadollahi M, Szojka A, Fekete E, Karaffa L, Takács F, Flipphi M, Sándor E (2013) Resistance to QoI fungicide and cytochrome b diversity in the Hungarian Botrytis cinerea population. J Agric Sci Technol 15:397–407
Bruce RJ, West CA (1989) Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures caster bean. Plant Physiol 91:889–897
Cawoy H, Mariutto M, Henry G, Fisher C, Vasilyeva N, Thonart P, Dommes J, Ongena M (2014) Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol Plant–Microbe Interact 27(2):87–100
Cawoy H, Debois D, Franzil L, De Pauw E, Thonart P, Ongena M (2015) Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotechnol 8(2):281–295
Chung S, Kong H, Buyer JS, Lakshman DK, Lydon J, Kim SD, Roberts DP (2008) Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soil borne pathogens of cucumber and pepper. Appl Microbiol Biotechnol 80:115–123
Daayf F, Schmitt A, Bélanger RR (1997) Evidence of phytoalexins in cucumber leaves infected with powdery mildew following treatment with leaf extracts of Reynoutria sachalinensis. Plant Physiol 113(3):719–727
Dennis C, Webster J (1971) Antagonistic properties of species group of Trichoderma production of non-volatile antibiotics. Trans Br Mycol Soc 57:25–39
Dheepa R, Vinodkumar S, Renukadevi P, Nakkeeran S (2016) Phenotypic and molecular characterization of chrysanthemum white rust pathogen Puccinia horiana (Henn) and the effect of liquid based formulation of Bacillus spp. for the management of Chrysanthemum white rust under protected cultivation. Biol Control 103:172–186
Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL (1984) Phenylalanine ammonia-lyase and hydroxy cinnamate CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Mol Plant Pathol 25:111–123
Durairaj K, Velmurugan P, Park J-H, Chang W-S, Park Y-J, Senthilkumar P, Choi K-M, Lee J-H, Oh B-T (2018) Characterization and assessment of two biocontrol bacteria against Pseudomonas syringae wilt in Solanum lycopersicum and its genetic responses. Microbiol Res 206:43–49
Fernando WGD, Ramarathnam R, Krishnamoorthy AS (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37:955–964
Gao X, Han Q, Chen Y, Qin H, Huang L, Kang Z (2014) Biological control of oilseed rape Sclerotinia stem rot by Bacillus subtilis strain Em7. Biocontrol Sci Technol 24(1):39–52
Gomez KA, Gomez AA (1984) Statistical procedure for agricultural research. Wiley, New York
Gorden SA, Paleg LG (1957) Quantitative measurements of indole acetic acid. Physiol Plant 4:24–27
Gruau C, Trotel-Aziz P, Villaume S, Rabenoelina F, Ci C, Baillieul F et al (2015) Pseudomonas fluorescens PTA-CT2 triggers local and systemic immune response against Botrytis cinerea in grapevine. Mol Plant–Microbe Interact 28:1117–1129
Hammerschmidt R, Nuckles EM, Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Mol Plant Pathol 20:73–82
Harish S, Kavino M, Kumar N, Balasubramanian P, Samiyappan R (2009) Induction of defense-related proteins by mixtures of plant growth promoting endophytic bacteria against Banana bunchy top virus. Biol Control 51:16–25
Hausbeck MK, Moorman GW (1996) Managing Botrytis in greenhouse-grown flower crops. Plant Dis 80:1212–1219
Henry GE, Momin RA, Nair MG, Dewitt DL (2002) Antioxidant and cyclooxygenase activities of fatty acids found in food. J Agric Food Chem 50(8):2231–2234
Indian Horticulture Database (2017) Statistical data. http://nhb.gov.in/statistics/Publication/Horticulture%20At%20a%20Glance%202017%20for%20net%20uplod%20(2).pdf
Jarvis WR (1980) Taxonomy. In: Coley-Smith JR, Verhoeff K, Jarvis WR (eds) The biology of Botrytis. Academic, London, pp 1–17
Ji SH (2013) Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 41(4):234–242
Jiang C, Liao M, Wang H, Zheng M, Xu J, Guo J (2018) Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol Control 126:147–157
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kefi A, Slimene B, Karkouch I, Rihouey C, Azaeiz S, Bejaoui M, Belaid R, Cosette P, Jouenne T, Limam F (2015) Characterization of endophytic Bacillus strains from tomato plants (Lycopersicon esculentum) displaying antifungal activity against Botrytis cinerea Pers. World J Microbiol Biotechnol 31(12):1967–1976
Khazaeli P, Zamanizadeh H, Morid B, Bayat H (2010) Morphological and molecular identification of Botrytis cinerea causal agent of gray mold in rose greenhouses in central regions of Iran. Int J Agric Sci Res 1:19–24
Kim YS, Song YG, Lee IK, Yeo WH, Yun BS (2013) Bacillus sp. BS061 suppresses powdery mildew and gray mold. Mycobiology 41(2):108–111
Li L, Steffens JC (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215:239–247
Mahadevan A, Sridhar R (1982) Methods in physiological plant pathology, 2nd edn. Sivakami Publishers, Madras, pp 24–44
Mayer AM, Harel E, Shaul RB (1965) Assay of catechol oxidase: a critical comparison of methods. Phytochemistry 5:783–789
Mohite B (2013) Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr 13(3):638–649
Montesinos E, Bonaterra A (2009) Microbial pesticides. M. Schaechter. In Encyclopedia of Microbiology, Ed. 3rd ed. Elsevier. Pp. 110–120
Mora I, Cabrefiga J, Montesinos E (2011) Antimicrobial peptide genes in Bacillus strains from plant environments. Int Microbiol 14:213–223
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64:548–572
Ongena M, Jacques P (2007) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16(3):115–125
Patel JS, Kharwar RN, Singh HB, Upadhyay RS, Sarma BK (2017) Trichoderma asperellum (T42) and Pseudomonas fluorescens (OKC)-enhances resistance of pea against Erysiphe pisi through enhanced ROS generation and lignifications. Front Microbiol 8:306
Pourbabaee AA, Bahmani E, Alikhani HA, Emami S (2016) Promotion of wheat growth under salt stress by halotolerant bacteria containing ACC deaminase. J Agric Sci Technol 18:855–864
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062
Radhakrishnan R, Hashem A, Abd Allah EF (2017) Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol 8:667
Rais A, Jabeen Z, Shair F, Hafeez FY, Hassan MN (2017) Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE 12(11):e0187412
Rajeswari G, Murugan M, Mohan VR (2012) GC–MS analysis of bioactive components of Hugonia mystax L. (Linaceae). Res J Pharm Biol Chem Sci 3(4):301–308
Reetha S, Bhuvaneswari G, Thamizhiniyan P, Ravi Mycin T (2014) Isolation of indole acetic acid (IAA) producing rhizobacteria of Pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allim cepa L.). Int J Curr Microbiol Appl Sci 3(2):568–574
Rimola A, Costa D, Sodupe M, Lambert JF, Ugliengo P (2013) Silica surface features and their role in the adsorption of bio-molecules: computational modeling and experiments. Chem Rev 4:1–12
Romero D, De Vicente A, Rakotoaly RH, Dufour SE, Veening JW, Arrebola E, Cazorla FM, Kuipers OP, Paquot M, Perez-Garcia A (2007) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant–Microbe Interact 20:430–440
Schroth MN, Hancock JG (1982) Disease-suppressive soil and root-colonizing bacteria. Science 216:1376–1381
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31(3):446–459
Siripornvisal S (2010) Biocontrol efficacy of Bacillus subtilis BCB3-19 against tomato gray mold. KMITL Sci Technol J 10(2):37–44
Srivastava S, Patel JS, Singh HB, Sinha A, Sarma BK (2015) Streptomyces rochei SM3 induces stress tolerance in chickpea against Sclerotinia sclerotiorum and NaCl. J Phytopathol 163:583–592
Tien TM, Gasking MH, Hubbel DH (1979) Plant growth substances produced by A. brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol 37:1016–1024
Van Loon LC (1997) Induced resistance in plant and the role of pathogenesis related protein. Eur J Plant Pathol 103:753–765
Vidhyasekaran P (2014) PAMP signals in plant innate immunity: signal perception and transduction. Springer, Dordrecht
Vinodkumar S, Nakkeeran S (2017) Characterization and management of Botrytis cinerea inciting blossom blight of carnation under protected cultivation. J Environ Biol 38(4):527–537
Vinodkumar S, Nakkeeran S (2018) Bacillus amyloliquefaciens (VB7) with diverse anti microbial peptide genes: a potential antagonist for the management of fairy ring spot in carnations. Curr Sci 115(8):1519–1524
Vinodkumar S, Nakkeeran S, Renukadevi P, Malathi VG (2017) Biocontrol potentials of antimicrobial peptide producing Bacillus species: multifaceted antagonists for the management of stem rot of carnation caused by Sclerotinia sclerotiorum. Front Microbiol 8:446
Vinodkumar S, Nakkeeran S, Renukadevi P, Mohankumar S (2018) Diversity and antiviral potential of rhizospheric and endophytic Bacillus species and phyto-antiviral principles against tobacco streak virus in cotton. Agric Ecosyst Environ 267:42–51
Wang X, Wang L, Wang J, Jin P, Liu H, Zheng Y (2014) Bacillus cereus AR156-induced resistance to Colletotrichum acutatum is associated with priming of defense responses in loquat fruit. PLoS ONE 9(11):e112494
Williamson B, Tudzynski B, Tudzynski P, van Kan J (2007) Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8:561–580
Xu SS, Friesen TL, Mujeeb-Kazi A (2004) Seedling resistance to tan spot and Stagonospora nodorum blotch in synthetic hexaploid wheats. Crop Sci 44:2238–2245
Xu M, Sheng J, Chen L, Men Y, Gan L, Guo S et al (2014) Bacterial community compositions of tomato (Lycopersicum esculentum Mill.) seeds and plant growth promoting activity of ACC deaminase producing Bacillus subtilis (HYT-12-1) on tomato seedlings. World J Microbiol Biotechnol 30:835–845
Yoshida S, Hiradate S, Tsukamoto T, Hatakeda K, Shirata A (2001) Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology 91:181–187
Zapata Y, Díaz A, Grijalba E, Rodríguez F, Elad Y, Cotes AM (2016) Phyllosphere yeasts with potential for biological control of Botrytis cinerea in rose. Acta Hortic 1144:77–84
Acknowledgements
The authors would like to acknowledge the support provided by Professor and Head (Department of Plant Pathology) and The Dean, School of Post Graduate Studies, Tamil Nadu Agricultural University. DST-FIST (Department of Science and Technology, New Delhi) and UGC-SAP are deeply acknowledged for providing infrastructure facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Researchgate: https://www.researchgate.net/profile/Vinodkumar_S/publications?pubType=article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakkeeran, S., Surya, T. & Vinodkumar, S. Antifungal Potential of Plant Growth Promoting Bacillus Species Against Blossom Blight of Rose. J Plant Growth Regul 39, 99–111 (2020). https://doi.org/10.1007/s00344-019-09966-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09966-1