Abstract
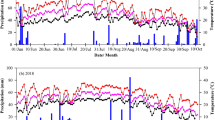
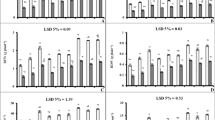
The degradation of photosynthetic pigments leads to leaf senescence and thus grain yield losses. We determined whether the application of uniconazole to maize in semiarid regions could reduce the degradation of photosynthetic pigments by enhancing the antioxidant defense system. We conducted a field study in the summers of 2015 and 2016 where seeds were soaked in uniconazole at concentrations of 0 (CK1), 25 (S1), 50 (S2), and 75 (S3) mg kg−1 and foliar sprayed at concentrations of 0 (CK2), 25 (F1), 50 (F2), and 75 (F3) mg L−1 at the eight-leaf stage. The application of uniconazole significantly improves the chlorophyll content, soluble protein content, and net photosynthetic rate, where the maximum values were obtained with the S1 and F1 treatments. Uniconazole significantly improved the activities of antioxidant enzymes comprising superoxide dismutase, peroxidase, and catalase, but reduced that of malondialdehyde (MDA) and the accumulation of reactive oxygen species (ROS) during the leaf senescence process. Treatments S1 and F1 had higher antioxidant enzyme activities but reduced MDA, superoxide radical, and hydrogen peroxide contents. Uniconazole significantly reduced leaf senescence in leaves in the bottom layer, while also increasing the middle layer leaf area and decreasing the top layer leaf area. The degradation of photosynthetic pigments was reduced by uniconazole because the enhanced antioxidant activities of enzymes protected plants from harmful ROS. Uniconazole significantly improved the photosynthetic traits, antioxidant defense system, and grain yield in maize in semiarid regions, where the most effective treatment was S1.










Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad I, Kamran M, Ali S, Bilegjargal B, Cai T, Ahmad S, Meng XP, Su WN, Liu T, Han QF (2018) Uniconazole application strategies to improve lignin biosynthesis, lodging resistance and production of maize in semiarid regions. Field Crops Res 222:66–77
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Aly A, Latif H (2011) Differential effects of paclobutrazol on water stress alleviation through electrolyte leakage, phytohormones, reduced glutathione and lipid peroxidation in some wheat genotypes (Triticum aestivum L.) grown in-vitro. Rom Biotechnol Lett 16:6710–6721
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 55:373–399
Asada K (1992) Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85:235–241
Bela K, Horváth E, Gallé Á, Szabados L, Tari I, Csiszár J (2015) Plant glutathione peroxidases: emerging role of the antioxidant enzymes in plant development and stress responses. J Plant Physiol 176:192–201
Bhattacharjee S (2014) Membrane lipid peroxidation and its conflict of interest: the two faces of oxidative stress. Curr Sci 107:1811–1823
Blokhina O, Virolainen E, Gagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Catherine MS (2010) Gene expression during leaf senescence. New Phytol 126:419–448
Dai HP, Zhang PP, Lu C, Jia GL, Song H, Ren XM, Chen J, Wei AZ, Feng BL, Zhang SQ (2011) Leaf senescence and reactive oxygen species metabolism of broomcorn millet (Panicum miliaceum L.) under drought condition. Aust J Crop Sci 5:1655–1660
Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 581:320–324
Davis DG, Swanson HR (2001) Activity of stress-related enzymes in the perennial weed leafy spurge (Euphorbia esula L.). Environ Exp Bot 46:95–108
Djanaguiraman M, Sheeba JA, Devi DD, Bangarusamy U (2009) Cotton leaf senescence can be delayed by nitrophenolate spray through enhanced antioxidant defence system. J Agron Crop Sci 195:213–224
Duan L, Guan C, Li J, Eneji AE, Li Z, Zhai Z (2008) Compensative effects of chemical regulation with uniconazole on physiological damages caused by water deficiency during the grain filling stage of wheat. J Agron Crop Sci 194:9–14
El Hadrami A, Kone D, Lepoivre P (2005) Effect of juglone on active oxygen species and antioxidant enzymes in susceptible and partially resistant banana cultivars to black leaf streak disease. Eur J Plant Pathol 113:241–254
Erley GSA, Ambebe TF, Worku M, Bänziger M, Horst WJ (2010) Photosynthesis and leaf-nitrogen dynamics during leaf senescence of tropical maize cultivars in hydroponics in relation to N efficiency in the field. Plant Soil 330:313–328
Fang X, Liu X, Zhang Y, Huang K, Zhang Y, Li Y, Nie J, She H, Ruan R, Yuan X, Yi Z (2018) Effects of uniconazole or gibberellic acid application on the lignin metabolism in relation to lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). J Agron Crop Sci 00:1–10
Finaud J, Lac G, Filaire E (2006) Oxidative stress: relationship with exercise training. Sports Med 36:327–359
Fletcher RA, Hofstra G (1990) Improvement of uniconazole induced protection in wheat seedlings. J Plant Growth Regul 9:207–212
Fletcher RA, Kallidumbil V, Steele P (1982) An improved bioassay for cytokinins using cucumber cotyledons. Plant Physiol 69:675–677
Fletcher RA, Gilley A, Davis TD, Sankhla N (2000) Triazole as plant growth regulators and stress protectants. Hort Rev 24:55–138
Ford TW, Simon EW (1972) Peroxidase and glucose-6-phosphate dehydrogenase levels in cotyledons of Cucumis sativus (L.). J Exp Bot 23:423–428
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gooding MJ, Dimmock JPRE, France J, Jones SA (2015) Green leaf area decline of wheat flag leaves: the influence of fungicides and relationships with mean grain weight and grain yield. Ann Appl Biol 136:77–84
Guoping Z, Jianxing C, Bull DA (2001) The effects of timing of N application and plant growth regulators on morphogenesis and yield formation in wheat. Plant Growth Regul 35:239–245
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Han H, Yang WY (2009) Influence of uniconazole and plant density on nitrogen content and grain quality in winter wheat in South China. Plant Soil Environ 55:159–166
Han LP, Wang XL, Guo XQ, Rao MS, Steinberger Y, Xu C, Xie GH (2011) Effects of plant growth regulators on growth, yield and lodging of sweet sorghum. Res Crops 12:372–382
Hernández JA, Jiménez A, Mullineaux P, Sevila F (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23:853–862
Huang MJ, Fang Y, Liu Y, Jin Y, Sun JL, Tao X, Ma XR, He KZ, Zhao H (2015) Using proteomic analysis to investigate uniconazole-induced phytohormone variation and starch accumulation in duckweed (Landoltia punctata). BMC Biotechnol 15:81
Hui Z, Tian FX, Wang GK, Wang GP, Wang W (2012) The antioxidative defense system is involved in the delayed senescence in a wheat mutant tasg1. Plant Cell Rep 31:1073–1084
Hussein MM, Bakheta MA, Zaki SNS (2014) Influence of uniconazole on growth characters, photosynthetic pigments, total carbohydrates and total soluble sugars of Hordium vulgare L plants grown under salinity stress. Int J Sci Res 3:2208–2213
Jaleel CA, Riadh K, Gopi R, Manivannan P, Ines J, Al-Juburi HJ, Chang-Xing Z, Hong-Bo S, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436
Kamran M, Cui W, Ahmad I, Meng XP, Zhang X, Su W, Chen J, Ahmad S, Fahad S, Han QF, Tiening L (2018a) Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize. Plant Growth Regul 84:317–332
Kamran M, Su W, Ahmad I, Xiangping M, Wenwen C, Xudong Z, Siwei M, Khan A, Qingfang H, Tiening L (2018b) Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Sci Rep 8:4818
Kamran M, Ahmad I, Wang HQ, Wu XR, Xu J, Tiening L, Ding RX, Han QF (2018c) Mepiquat chloride application increases lodging resistance of maize by enhancing stem physical strength and lignin biosynthesis. Field Crops Res 224:148–159
Kariali E, Mohapatra PK (2007) Hormonal regulation of tiller dynamics in differentially-tillering rice cultivars. Plant Growth Regul 53:215–223
Karpinski S, Muhlenbock P (2007) Genetic, molecular and physiological mechanisms controlling cell death, defenses, and antioxidant network in response to abiotic and biotic stresses in plants. Comp Biochem Physiol A 146:60–66
Khalil A, Rahman H (1995) Effect of paclobutrazol on growth, chloroplast pigments and sterol biosynthesis of maize (Zea mays L.). Plant Sci 105:15–21
Kim HY, Choi BJ, Sang CK (1994) Effects of uniconazole on the drought resistance of Pilea cadierei. II. Physiological changes and drought resistance. J Korean Soc Hort Sci 35:493–498
Kraus TE, Murr DP, Fletcher RA (1991) Uniconazole inhibits stress-induced ethylene in wheat and soybean seedlings. J Plant Growth Regul 10:229–234
Ku JH, Drizek DT, Mirecki RM (1996) Alleviation of sulfur dioxide injury in snap bean plants by uniconazole. J Korean Soc Hort Sci 37:767–772
Kukavica B, Jovanovic SV (2004) Senescence-related changes in the antioxidant status of ginkgo and birch leaves during autumn yellowing. Physiol Plant 122:321–327
Leul M, Zhou WJ (1998a) Alleviation of waterlogging damage in winter rape by application of uniconazole, effects on morphological characteristics, hormones and photosynthesis. Field Crops Res 59:121–127
Leul M, Zhou WJ (1998b) Alleviation of waterlogging damage in winter rape by uniconazole application: effects on enzyme activity, lipid peroxidation, and membrane integrity. J Plant Growth Regul 18:9–14
Li ZZ, Niu W, Qiao XW, Ma LP (2007) Anti-oxidant response of Cucumis sativus L. to fungicide carbendazim. Pest Biochem Physiol 89:54–59
Lin ZF, Li SX, Lin GZ, Guo GZ (1988) Relationship between cumulation of H2O2 and membrane lipid peroxidation in senescent leaf and chloroplast. Acta Photophysiol Sin 14:16–22
Liu XZ, Huang BR (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510
Liu J, Yeo HC, Doniger SJ, Ames BN (1997) Assay of aldehydes from lipid peroxidation: gas chromatograph-mass spectrometry compared to thiobarbituric acid. Anal Biochem 245:161–166
Liu Y, Fang Y, Huang M, Jin Y, Sun J, Tao X, Zhang G, He K, Zhao Y, Zhao H (2015a) Uniconazole-induced starch accumulation in the bioenergy crop duckweed (Landoltia punctata) I: transcriptome analysis of the effects of uniconazole on chlorophyll and endogenous hormone biosynthesis. Biotechnol Biofuels 8:57
Liu Y, Fang Y, Huang MJ, Jin YL, Sun JL, Tao X, Zhang GH, He KZ, Zhao Y, Zhao H (2015b) Uniconazole-induced starch accumulation in the bioenergy crop duckweed (Landoltia punctata) II: transcriptome alterations of pathways involved in carbohydrate metabolism and endogenous hormone crosstalk. Biotechnol Biofuels 8:57
Lu W, Xu XM, Zhang RX, Dai XB (2004) Effect of adding acetic acid on improvement of determination of superoxide anion content in plants. J N Norm Univ 27:82–84
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Gollery M, Breusegem FV (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Nooden L (1988) Whole plant senescence. In: Nooden LD, Leopold AC (eds) Senescence and aging in plants. Academic Press, San Diego, pp 391–439
Nouriyani H, Majidi E, Seyyednejad SM, Siadat SA, Naderi A (2012) Effect of paclobutrazol under different levels of nitrogen on some physiological traits of two wheat cultivars (Triticum aestivum L.). World Appl Sci J 16:1–6
Pan S, Rasul F, Wu L, Hua T, Mo Z, Duan M, Tang X (2013) Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 6:9
Pastori GM, Del Rio LA (1997) Natural senescence of pea leaves (an activated oxygen-mediated function for peroxisomes). Plant Physiol 113:411–418
Percival C, Albalushi M (2007) Paclobutrazol-induced drought tolerance in containerized english and evergreen oak. Arboricult Urban For 33:397–409
Qiu J, Wang RM, Yan JZ, Hu J (2005) Seed film coating with uniconazole improves rape seedling growth in relation to physiological changes under waterlogging stress. Plant Growth Regul 47:75–81
Quirino BF, Yoosun N, Himelblau E, Amasino RM (2000) Molecular aspects of leaf senescence. Trends Plant Sci 5:278–282
Rajcan I, Dwyer LM, Tollenaar M (1999) Note on relationship between leaf soluble carbohydrate and chlorophyll concentrations in maize during leaf senescence. Field Crops Res 63:13–17
Sang HL, Ahsan N, Lee KW, Kim DH, Lee DG, Kwak SS, Kwon SY, Kim TH, Lee BH (2007) Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol 64:1626–1638
Sankar B, Jaleel CA, Manivannan P, Kishorekumar A, Somasundaram R, Panneeelvam R (2007) Effect of paclobutrazol on water stress amelioration through antioxidants and free radical scavenging enzymes in Arachis hypogaea L.. Colloids Surf B 60:229–235
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Scandalios JG (1997) Molecular genetics of SOD in plants. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defense. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 527–568
Schluttenhofer CM, Massa GD, Mitchell CA (2011) Use of uniconazole to control plant height for an industrial/pharmaceutical maize platform. Ind Crops Prod 33:720–726
Shao HB, Liang ZS, Shao MA (2005) Changes of anti-oxidative enzymes and MDA content under soil water deficits among10 wheat (Triticum aestivum L.) genotypes at maturation stage. Colloids Surf B 45:7–13
Somasundaram R, Jaleel CA, Sindhu SA, Azooz MM, Panneerselvam R (2009) Role of Paclobutrazol and ABA in drought stress amelioration in Sesamum indicum L. Glob J Mol Sci 4:56–62
Spanic V, Vuletic MJ, Abicic I, Marcek T (2017) Early response of wheat antioxidant system with special reference to Fusarium head blight stress. Plant Physiol Biochem 115:34–43
Thompson JE, Legge RL, Barber RF (1987) The role of free radicals in senescence and wounding. New Phytol 105:317–344
Upadhyaya A, Davis TD, Larsen MH, Walser RH, Sankhla N (1990) Uniconazole-induced thermotolerance in soybean seedling root tissue. Physiol Plant 79:78–84
Wang AG, Luo GH (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun 84:2895–2898
Wang XC, Yang WY, Chen G, Qian-Liang LI, Wang XB (2009) Effects of uniconazole on leaf senescence and yield of maize sprayed at late growth stage. J Maize Sci 17:86–88
Wang C, Hu D, Liu X, She H, Ruan R, Yang H, Yi Z, Wu D (2015a) Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crops Res 180:46–53
Wang YC, Gu WR, Ye LF, Sun Y, Jie LL, Zhang H, Li J, Wei S (2015b) Physiological mechansim of delaying leaf senescence in maize treated with compound mixtures of DCPTA and CCC. J Northeast Agric Univ 22:1–15
Wang F, Liu J, Zhou L, Pan G, Li Z, Zaidi SH, Cheng F (2016) Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves. Plant Physiol Biochem 109:248–261
Wu YX, Tiedemann AV (2001) Physiological effects of azoxystrobin and epoxiconazole on senescence and the oxidative status of wheat. Pest Biochem Physiol 71:1–10
Yamori W, Noguchi K, Hikosaka K, Terashima I (2010) Phenotypic plasticity in photosynthetic temperature acclimation among crop species with different cold tolerances~(l[W][O]). Plant Physiol 152:388–399
Yan YH, Yan W, Liu WG, Wang XC, Yong TW, Yang WY, Zhao LL (2015) Influence of seed treatment with uniconazole powder on soybean growth, photosynthesis, dry matter accumulation after flowering and yield in relay strip intercropping system. Plant Prod Sci 18:295–301
Zhang ZL (2001) Experimental guide of plant physiology. Higher Education Press, Beijing
Zhang MC, Duan LS, Tian XL, He ZP, Li JM, Wang BM, Li ZH (2007) Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis, hormones and antioxidant system. J Plant Physiol 164:709–717
Zhang YJ, Zhang X, Chen CJ, Zhou MG, Wang HC (2010) Effects of fungicides JS399-19, azoxystrobin, tebuconazloe, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pest Biochem Physiol 98:151–157
Zhang J, Cao XL, Yong TW, Yang WY (2012) Seed treatment with uniconazole powder induced drought tolerance of soybean in relation to changes in photosynthesis and chlorophyll fluorescence. Res Crops 13:147–154
Zhang Q, Zhang L, Evers J, Werf WVD, Zhang W, Duan L (2014) Maize yield and quality in response to plant density and application of a novel plant growth regulator. Field Crops Res 164:82–89
Zhao H, Dai T, Jing Q, Jiang D, Cao W (2007) Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul 51:149–158
Zhou W, Ye Q (1996) Physiological and yield effects of uniconazole on winter rape (Brassica napus L.). J Plant Growth Regul 15:69–73
Acknowledgements
The authors extend their sincere thanks to the editors of this journal and the anonymous reviewers for their valuable comments and suggestions that have significantly improved the manuscript. We are also grateful to Ding Ruixia, Nie Junfeng, and Yang Baoping for help during experimental period.
Funding
This study was supported by funding from High Technology Research and Development Program of China (863 Program, No.2013AA102902), the National Natural Science Foundation of China (No. 31601256), the special fund for Agro-scientific Research in the Public Interest (201303104), the 111 Project of Chinese Education Ministry (B12007).
Author information
Authors and Affiliations
Contributions
QH, LT, and TC conceived and designed the research. IA performed research. MK, WX, SA, and SA contributed in the field experiments. IA wrote the manuscript. BB helped in English revision. WS helped in revision of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahmad, I., Kamran, M., Su, W. et al. Application of Uniconazole Improves Photosynthetic Efficiency of Maize by Enhancing the Antioxidant Defense Mechanism and Delaying Leaf Senescence in Semiarid Regions. J Plant Growth Regul 38, 855–869 (2019). https://doi.org/10.1007/s00344-018-9897-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9897-5