Abstract
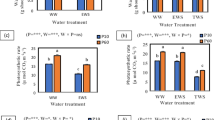
We evaluated the effect of different watering regimes on the growth, chlorophyll fluorescence, phytohormones, and phenolic acids in Ceratotheca triloba (Bernh.) Hook.f., a commonly consumed African indigenous leafy vegetable. The study was conducted in the greenhouse under different watering regimes [seven (daily); three (thrice); two (twice); one (once) day(s) per week] for a period of 2 and 4-months. In each pot (7.5 cm diameter; 150 ml volume), 50 ml of water was applied per treatment. At the end of the experiment, plant growth, chlorophyll fluorescence, phytohormones, and phenolic acids were determined. A decrease in water availability resulted in a consistent decline in plant growth after a 4-month growth period. The severity of reduced water availability was more noticeable in plants watered once a week with a 1.4-fold reduction in growth and quantum efficiency of PSII (Fv/Fm) value of 0.80. The significant decline in growth and chlorophyll fluorescence was probably due to the increased production of abscisic acid (ABA) and cytokinin (CK) content together with the detected phytohormones in plants with restricted water supply. Furthermore, plants watered once a week had a trade-off between growth and phenolic acid production, with significantly higher (threefolds) concentrations of vanillic, ferulic, caffeic, and 4-coumaric acids in 4-month-old plants. Even though C. triloba grew best in well-watered soil, the plant had the potential to adapt and survive in soils with limited water supply for longer periods of growth. These findings suggest that regulation of phytohormones and phenolic acids played an important role in improving the growth of C. triloba under limited water conditions.





Similar content being viewed by others
References
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Anjum SA, Xie X-y, Wang L-c, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6:2026–2032
Bacelar EA, Moutinho-Pereira JM, Gonçalves BC, Ferreira HF, Correia CM (2007) Changes in growth, gas exchange, xylem hydraulic properties and water use efficiency of three olive cultivars under contrasting water availability regimes. Environ Exp Bot 60:183–192
Bajguz A, Piotrowska A (2009) Conjugates of auxin and cytokinin. Phytochemistry 70:957–969
Bano A, Yasmeen S (2010) Role of phytohormones under induced drought stress in wheat. Pak J Bot 42:2579–2587
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Beckett RP, Marschall M, Laufer Z (2005) Hardening enhances photoprotection in the moss Atrichum androgynum during rehydration by increasing fast-rather than slow-relaxing quenching. J Bryol 27:7–12
Behr M, Motyka V, Weihmann F, Malbeck J, Deising HB, Wirsel SG (2012) Remodeling of cytokinin metabolism at infection sites of Colletotrichum graminicola on maize leaves. Mol Plant Microbe Interact 25:1073–1082
Bengough AG, McKenzie BM, Hallett PD, Valentine TA (2011) Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J Exp Bot 62:59–68
Brzobohaty B, Moore I, Kristoffersen P, Bako L, Campos N, Schell J, Palme K (1993) Release of active cytokinin by a β-glucosidase localized to the maize root meristem. Science 262:1051–1054
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S (2013) Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem 72:1–20
Depuydt S, Hardtke CS (2011) Hormone signalling crosstalk in plant growth regulation. Curr Biol 21:R365–R373
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Du H, Liu H, Xiong L (2013) Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci. https://doi.org/10.3389/fpls.2013.00397
Edreva A, Velikova V, Tsonev T, Dagnon S, Gürel A, Aktaş L, Gesheva E (2008) Stress-protective role of secondary metabolites: diversity of functions and mechanisms. Gen Appl Plant Physiol 34:67–78
Floková K, Tarkowská D, Miersch O, Strnad M, Wasternack C, Novák O (2014) UHPLC-MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 105:147–157
Gajdošová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, Galuszka P, Klíma P, Gaudinová A, Žižková E, Hanuš J, Dančák M, Trávníček B, Pešek B, Krupička M, Vaňková R, Strnad M, Motyka V (2011) Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot 62:2827–2840
Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S (2013) Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol 161:2159–2170
Grúz J, Novák O, Strnad M (2008) Rapid analysis of phenolic acids in beverages by UPLC–MS/MS. Food Chem 111:789–794
Gutierrez L, Mongelard G, Floková K, Păcurar DI, Novák O, Staswick P, Kowalczyk M, Păcurar M, Demailly H, Geiss G, Bellini C (2012) Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 24:2515–2527
Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran L-SP (2012) Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci 17:172–179
Johnson GN, Young AJ, Scholes JD, Horton P (1993) The dissipation of excess excitation energy in British plant species. Plant Cell Environ 16:673–679
Khan MIR, Khan NA (2013) Salicylic acid and jasmonates: approaches in abiotic stress tolerance. J Plant Biochem Physiol. https://doi.org/10.4172/2329-9029.1000e113
Kohli A, Sreenivasulu N, Lakshmanan P, Kumar PP (2013) The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep 32:945–957
Kroon PA, Williamson G (1999) Hydroxycinnamates in plants and food: current and future perspectives. J Sci Food Agric 79:355–361
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Liberato MAR, Gonçalves JFDC, Chevreuil LR, Junior N, da Rocha A, Fernandes AV, Santos Junior UMD (2006) Leaf water potential, gas exchange and chlorophyll a fluorescence in acariquara seedlings (Minquartia guianensis Aubl.) under water stress and recovery. Braz J Plant Physiol 18:315–323
Lu C, Zhang J (1999) Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J Exp Bot 50:1199–1206
Masondo NA, Finnie JF, Van Staden J (2016a) Nutritional and pharmacological potential of the genus Ceratotheca: an underutilized leafy vegetable of Africa. J Ethnopharmacol 178:209–221
Masondo NA, Kulkarni MG, Rengasamy KRR, Pendota SC, Finnie JF, Van Staden J (2016b) Effect of vermicompost leachate in Ceratotheca triloba under nutrient deficiency. Acta Physiol Plant. https://doi.org/10.1007/s11738-016-2252-1
Masondo NA, Kulkarni MG, Finnie JF, Van Staden J (2018) Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmotic potential and salinity stress. Ecotoxicol Environ Saf 147:43–48
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51:659–668
Miura K, Okamoto H, Okuma E, Shiba H, Kamada H, Hasegawa PM, Murata Y (2013) SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J 73:91–104
Munné-Bosch S, Peñuelas J (2003) Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 217:758–766
Naczk M, Shahidi F (2006) Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J Pharm Biom Anal 41:1523–1542
Novák O, Hauserová E, Amakorová P, Doležal K, Strnad M (2008) Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69:2214–2224
O’Brien J, Benkova E (2013) Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci. https://doi.org/10.3389/fpls.2013.00451
Odhav B, Beekrum S, Akula U, Baijnath H (2007) Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J Food Compost Anal 20:430–435
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Plačková L, Hrdlička J, Smýkalová I, Cvečková M, Novák O, Griga M, Doležal K (2015) Cytokinin profiling of long-term in vitro pea (Pisum sativum L.) shoot cultures. Plant Growth Regul 77:125–132
Schäfer M, Brütting C, Meza-Canales ID, Großkinsky DK, Vankova R, Baldwin IT, Meldau S (2015) The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J Exp Bot 66:4873–4884
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics*. J Exp Bot 55:2343–2351
Souza RP, Machado EC, Silva JAB, Lagôa AMMA, Silveira JAG (2004) Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ Exp Bot 51:45–56
Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A (2012) Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506:265–273
Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539
Wang C, Yang A, Yin H, Zhang J (2008) Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. J Integr Plant Biol 50:427–434
Wilkinson S, Kudoyarova GR, Veselov DS, Arkhipova TN, Davies WJ (2012) Plant hormone interactions: Innovative targets for crop breeding and management. J Exp Bot 63:3499–3509
Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001) Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol 127:315–323
Zaicovski CB, Zimmerman T, Nora L, Nora FR, Silva JA, Rombaldi CV (2008) Water stress increases cytokinin biosynthesis and delays postharvest yellowing of broccoli florets. Postharvest Biol Technol 49:436–439
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res 97:111–119
Acknowledgements
We appreciate the financial support from the National Research Foundation (NRF, Grant UID: 89290), Graça Machel (Canon Collins) and the University of KwaZulu-Natal (Pietermaritzburg), South Africa. NAM and AOA thank the Stellenbosch University and North West University (Mmabatho) for provision of access to resources utilized during the preparation and revision of the manuscript. The Ministry of Education, Youth and Sport of the Czech Republic via National Program for Sustainability (Grant LO1204) and the Czech Science Foundation (Grant 14-17-06613S) are also thanked for their financial assistance. Prof R.P. Beckett (University of KwaZulu-Natal, Pietermaritzburg) is thanked for his assistance with chlorophyll fluorescence experiments.
Author information
Authors and Affiliations
Contributions
NAM conceived the research idea, designed the study, conducted greenhouse experiments and quantified stress-related phytohormones. AOA, MGK, ON, JG, KD advised on relevant experimental design and experiments performed. JG as well as KD and MS provided equipment used for quantification of phenolic acids and phytohormones, respectively. AOA, IP, LP and MŠ assisted with the quantification of phytohormones. AOA provided technical assistance in the interpretation of the phytohormone and phenolic acid data. NAM prepared the manuscript while all authors edited and approved the final version. JFF and JVS coordinated and supervised the project.
Corresponding author
Rights and permissions
About this article
Cite this article
Masondo, N.A., Aremu, A.O., Kulkarni, M.G. et al. How Do Different Watering Regimes Affect the Growth, Chlorophyll Fluorescence, Phytohormone, and Phenolic Acid Content of Greenhouse-Grown Ceratotheca triloba?. J Plant Growth Regul 38, 385–399 (2019). https://doi.org/10.1007/s00344-018-9848-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9848-1