Abstract
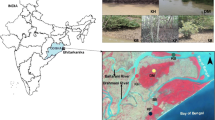
Bacteria are important regulators of carbon cycling in lakes and are central to sediment ecosystem processes. However, the sediment microbial communities and their respiratory responses to the lake wetland succession are poorly understood. In this study, we collected sediment samples from four different succession points (the Potamogeton lucens zone, the Scirpus tabernaemontani zone, the Scirpus triqueter zone, and the Juncus effusus zone) in the Caohai Wetland of the Guizhou Plateau (China). The bacterial communities at these succession points were studied using a high-throughput sequencing approach. The sediment microbial respiration (SR) was measured using static chambers in the field and basal respiration (BR) was determined in the laboratory. The results show that the dominant bacterial taxa in the sediment was Proteobacteria (34.7%), Chloroflexi (17.8%), Bacteroidetes (7.3%), Acidobacteria (6.6%), and Cyanobacteria (6.1%). Principal coordinate analysis showed that the microbial community structure differs significantly at different sampling points along the successional gradient, indicating that the bacterial community structure is sensitive to the lake wetland succession. Different hydrological regimes and soil characteristics such as \(\rm{NH}_4^+-N\), Fe2+, Mn2+, and sediment organic carbon (SOC) content may be important factors responsible for the differences in the sediment microbial characteristics of the different successional stages in the Caohai wetland. Additionally, it was found that the SR increased significantly from the P. lucens zone to the J. effusus zone, but BR had the opposite response. The shifts in the bacterial community structure along the successional gradient may be the main reason for the observed differences in sediment respiration.
Similar content being viewed by others
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.
References
Amato K R, Yeoman C J, Kent A, Righini N, Carbonero F, Estrada A, Gaskins H R, Stumpf R M, Yildirim S, Torralba M, Gillis M, Wilson B A, Nelson K E, White B A, Leigh S R. 2013. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. The International Society for Microbial Ecology Journal, 7: 1 344–1 353, https://doi.org/10.1038/ismej.2013.16.
Balasooriya W K, Denef K, Peters J, Verhoest N E C, Boeckx P. 2008. Vegetation composition and soil microbial community structural changes along a wetland hydrological gradient. Hydrology and Earth System Sciences Discussions, 12(1): 277–291, https://doi.org/10.5194/hess-12-277-2008.
Bond-Lamberty B, Thomson A. 2010. Temperature-associated increases in the global soil respiration record. Nature, 464(7288): 579–582, https://doi.org/10.1038/nature08930.
Bulseco A N, Vineis J H, Murphy A E, Spivak A C, Giblin A E, Tucker J, Bowen J L. 2020. Metagenomics coupled with biogeochemical rates measurements provide evidence that nitrate addition stimulates respiration in salt marsh sediments. Limnology and Oceanography, 65(S1): S321–S339, https://doi.org/1002/lno.1132.
Burns J H, Anacker B L, Strauss S Y, Burke D J. 2015. Soil microbial community variation correlates most strongly with plant species identity, followed by soil chemistry, spatial location and plant genus. AOB Plants, 7(1): plv030, https://doi.org/10.1093/aobpla/plv030.
Chen H Y, Zou J Y, Cui J, Nie M, Fang C M. 2018. Wetland drying increases the temperature sensitivity of soil respiration. Soil Biology and Biochemistry, 120: 24–27, https://doi.org/10.1016/j.soilbio.2018.01.035.
Chen J, Xie H J, Zhuang X L, Zhuang G Q, Bai Z H, Zhuang H X. 2008. Substrate-induced changes in microbial community-level physiological profiles and their application to discriminate soil microbial communities. Journal of Environmental Sciences, 20(6): 725–731, https://doi.org/10.1016/s1001-0742(08)62119-1.
Cook B I, Smerdon J E, Seager R, Coats S. 2014. Global warming and 21st century drying. Climate Dynamics, 43(9): 2 607–2 627, https://doi.org/10.1007/s00382-014-2075-y.
Edgar R C, Haas B J, Clemente J C, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27: 2 194–2 200, https://doi.org/10.1093/bioinformatics/btr381.
Eilers K G, Lauber C L, Knight R, Fierer N. 2010. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biology and Biochemistry, 42(6): 896–903, https://doi.org/10.1016/j.soilbio.2010.02.003.
Fenner N, Freeman C. 2011. Drought-induced carbon loss in peatlands. Nature Geoscience, 4(12): 895–900, https://doi.org/10.1038/ngeo1323.
Garbeva P V, Van Veen J A, Van Elsas J D. 2004. Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology, 42: 243–270, https://doi.org/10.1146/annurev.phyto.42.012604.135455.
Guo G X, Kong W D, Liu J B, Zhao J X, Du H D, Zhang X Z, Xia P H. 2015. Diversity and distribution of autotrophic microbial community along environmental gradients in grassland soils on the Tibetan Plateau. Applied Microbiology and Biotechnology, 99(20): 8 765–8 776, https://doi.org/10.1007/s00253-015-6723-x.
Jaatinen K, Laiho R, Vuorenmaa A, Del Castillo U, Minkkinen K, Pennanen T, Penttilä T, Fritze H. 2008. Responses of aerobic microbial communities and soil respiration to water-level drawdown in a northern boreal fen. Environmental Microbiology, 10(2): 339–353, https://doi.org/10.1111/j.1462-2920.2007.01455.x.
Jeanbille M, Buée M, Bach C, Cébron A, Frey-Klett P, Turpault M P, Uroz S. 2016. Soil parameters drive the structure, diversity and metabolic potentials of the bacterial communities across temperate beech forest soil sequences. Microbial Ecology, 71(2): 482–493, https://doi.org/10.1007/s00248-015-0669-5.
Jiang X T, Peng X, Deng G H, Sheng H F, Wang Y, Zhou H W, Tam N F Y. 2013. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microbial Ecology, 66(1): 96–104, https://doi.org/10.1007/s00248-013-0238-8.
Kayranli B, Scholz M, Mustafa A, Hedmark Å. 2010. Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands, 30(1): 111–124, https://doi.org/10.1007/s13157-009-0003-4.
Kraigher B, Stres B, Hacin J, Ausec L, Mahne I, Van Elsas J D, Mandic-Mulec I. 2006. Microbial activity and community structure in two drained fen soils in the Ljubljana Marsh. Soil Biology and Biochemistry, 38(9): 2 762–2 771, https://doi.org/10.1016/j.soilbio.2006.04.031.
Laiho R. 2006. Decomposition in peatlands: reconciling seemingly contrasting results on the impacts of lowered water levels. Soil Biology and Biochemistry, 38(8): 2 011–2 024, https://doi.org/10.1016/j.soilbio.2006.02.017.
Li Y L, Wang L, Zhang W Q, Wang H L, Fu X H, Le Y Q. 2011. The variability of soil microbial community composition of different types of tidal wetland in Chongming Dongtan and its effect on soil microbial respiration. Ecological Engineering, 37(9): 1 276–1 282, https://doi.org/10.1016/j.ecoleng.2011.03.024.
Liu C L, Xie G D, Huang H Q. 2006. Shrinking and drying up of Baiyangdian Lake wetland: a natural or human cause? Chinese Geographical Science, 16(4): 314–319, https://doi.org/10.1007/s11769-006-0314-9.
Ma Y T, Li J Q, Wu J, Kong Z Y, Feinstein L M, Ding X, Ge G, Wu L. 2018. Bacterial and fungal community composition and functional activity associated with lake wetland water level gradients. Scientific Reports, 8: 760, https://doi.org/10.1038/s41598-018-19153-z.
Mäkiranta P, Laiho R, Fritze H, Hytönen J, Laine J, Minkkinen K. 2009. Indirect regulation of heterotrophic peat soil respiration by water level via microbial community structure and temperature sensitivity. Soil Biology and Biochemistry, 41(4): 695–703, https://doi.org/10.1016/j.soilbio.2009.01.004.
Mayr M J, Besemer K, Sieczko A, Demeter K, Peduzzi P. 2020. Bacterial community composition and function along spatiotemporal connectivity gradients in the Danube floodplain (Vienna, Austria). Aquatic Sciences, 82: 28, https://doi.org/10.1007/s00027-020-0700-x.
Mitchell R J, Hester A J, Campbell C D, Chapman S J, Cameron C M, Hewison R L, Potts J M. 2010. Is vegetation composition or soil chemistry the best predictor of the soil microbial community? Plant and Soil, 333(1): 417–430, https://doi.org/10.1007/s11104-010-0357-7.
Noormets A, Gavazzi M J, Mcnulty S G, Domec J C, Sun G, King J S, Chen J Q. 2010. Response of carbon fluxes to drought in a coastal plain loblolly pine forest. Global Change Biology, 16(1): 272–287, https://doi.org/10.1111/j.1365-2486.2009.01928.x.
Planas-Clarke A M, Chimner R A, Hribljan J A, Lilleskov E A, Fuentealba B. 2020. The effect of water table levels and short-term ditch restoration on mountain peatland carbon cycling in the Cordillera Blanca, Peru. Wetlands Ecology and Management, 28(1): 51–69, https://doi.org/10.1007/s11273-019-09694-z.
Rui J P, Li J B, Wang S P, An J X, Liu W T, Lin Q Y, Yang Y F, He Z L, Li X Z. 2015. Responses of bacterial communities to simulated climate changes in alpine meadow soil of the qinghai-tibet plateau. Applied & Environmental Microbiology, 81: 6 070–6 077, https://doi.org/10.1128/AEM.02161-15.
Sato K, Kato Y, Taguchi G, Nogawa M, Yokota A, Shimosaka M. 2009. Chitiniphilus shinanonensis gen. nov., sp. nov., a novel chitin-degrading bacterium belonging to Betaproteobacteria. The Journal of General and Applied Microbiology, 55(2): 147–153, https://doi.org/10.2323/jgam.55.147.
Schiller V, Datry D, Corti T, Foulquier R, Tockner A, Marce K. 2019. Sediment respiration pulses in intermittent rivers and ephemeral streams. Global Biogeochemical Cycles, 33(10): 1 251–1 263, https://doi.org/10.1029/2019GB006276.
Schulze E D, Freibauer A. 2005. Carbon unlocked from soils. Nature, 437(7056): 205–206, https://doi.org/10.1038/437205a.
Sun R G, Yang J, Xia P H, Wu S L, Lin T, Yi Y. 2020. Contamination features and ecological risks of heavy metals in the farmland along shoreline of Caohai plateau wetland, China. Chemosphere, 254: 126828, https://doi.org/10.1016/j.chemosphere.2020.126828.
Tang Y S, Wang L, Jia J W, Fu X H, Le Y Q, Chen X Z, Sun Y 2011a. Response of soil microbial community in Jiuduansha wetland to different successional stages and its implications for soil microbial respiration and carbon turnover. Soil Biology and Biochemistry, 43(3): 638–646, https://doi.org/10.1016/j.soilbio.2010.11.035.
Tang Y S, Wang L, Jia J W, Li Y L, Zhang W Q, Wang H L, Sun Y. 2011b. Response of soil microbial respiration of tidal wetlands in the Yangtze River Estuary to different artificial disturbances. Ecological Engineering, 37(11): 1 638–1 646, https://doi.org/10.1016/j.ecoleng.2011.06.004.
Wang H J, Richardson C J, Ho M. 2015. Dual controls on carbon loss during drought in peatlands. Nature Climate Change, 5(6): 584–587, https://doi.org/10.1038/nclimate2643.
Wu X W, Cao R, Wei X, Xi X Q, Shi P L, Eisenhauer N, Sun S C. 2017. Soil drainage facilitates earthworm invasion and subsequent carbon loss from peatland soil. Journal of Applied Ecology, 54(5): 1 291–1 300, https://doi.org/10.1111/1365-2664.12894.
Xia P H, Kou Y Z, Yu L F. 2015. Carbon metabolic soil microbial community in Caohai Karst Plateau degraded wetland: a case study in southwest China. Acta Scientiae Circumstantiae, 35(8): 2 549–2 555, https://doi.org/10.13671/j.hjkxxb.2014.1010. (in Chinese with English abstract)
Xia P H, Yan D B, Sun R G, Song X, Lin T, Yi Y. 2020. Community composition and correlations between bacteria and algae within epiphytic biofilms on submerged macrophytes in a plateau lake, southwest China. Science of the Total Environment, 727: 138398, https://doi.org/10.1016/j.scitotenv.2020.138398.
Xiong J B, Liu Y Q, Lin X G, Zhang H Y, Zeng J, Hou J Z, Yang Y P, Yao T D, Knight R, Chu H Y. 2012. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environmental Microbiology, 14(9): 2457–2466,https://doi.org/10.1111/j.1462-2920.2012.02799.x.
Yang H C, Im W T, An D S, Park W S, Kim I S, Lee S T. 2005. Silvimonas terrae gen. nov., sp. nov., a novel chitin-degrading facultative anaerobe belonging to the ‘Betaproteobacteria’. International Journal of Systematic and Evolutionary Microbiology, 55(6): 2 329–2 332, https://doi.org/10.1099/ijs.0.63837-0.
Yin X J, Lu J, Wang Y C, Liu G L, Hua Y M, Wan X Q, Zhao J W, Zhu D W. 2020. The abundance of nirS-type denitrifiers and anammox bacteria in rhizospheres was affected by the organic acids secreted from roots of submerged macrophytes. Chemosphere, 240: 124903, https://doi.org/10.1016/j.chemosphere.2019.124903.
Zhang Y N, Li Y L, Wang L, Tang Y S, Chen J H, Hu Y, Fu X H, Le Y Q. 2013. Soil microbiological variability under different successional stages of the Chongming Dongtan wetland and its effect on soil organic carbon storage. Ecological Engineering, 52: 308–315, https://doi.org/10.1016/j.ecoleng.2012.10.002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Nos. 41867056, 31660150), the Construction Program of Biology First-class Discipline in Guizhou (No. GNYL[2017]009), the Joint Fund of the National Natural Science Foundation of China, and the Karst Science Research Center of Guizhou Province (No. U1812401)
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xia, P., Zhang, J., Liu, J. et al. Shifts of sediment bacterial community and respiration along a successional gradient in a typical karst plateau lake wetland (China). J. Ocean. Limnol. 39, 880–891 (2021). https://doi.org/10.1007/s00343-020-0073-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-020-0073-y