Abstract
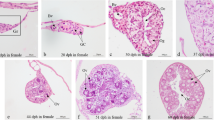
The aim of the present study was to investigate the long-term effects of 17β-estradiol (E2) exposure on gonadal development in the tiger puff er (Takifugu rubripes), which has a genetic sex determination system of male homogametic XY-XX. Tiger puff er larvae were exposed to 1, 10 and 100 μg/L E2 from 15 to 100 days post-hatch (dph) and then maintained in clean seawater until 400 dph. Changes in sex ratio, gonadal structure and gonadosomatic index (GSI) were monitored at 100, 160, 270 and 400 dph. Sex-associated single nucleotide polymorphism (SNP) markers were used to analyze the genetic sex of samples, except those at 100 dph. Exposure had a positive effect on the conversion of genetically male gonads into phenotypically female gonads at 100 dph. However, gonads from 60% of genetic XY males in the 1-μg/L E2 group and 100% in the 10-μg/L E2 group developed intersexual gonads at 160 dph; gonads of all genetic XY males in the two treatment groups reverted to testis by 270 dph. While 38%, 57% and 44% of gonads of XY fish in the 100-μg/L E2 group reverted to intersexual gonads at 160, 270 and 400 dph, respectively, none reverted to testis after E2 treatment. In addition, E2 exposure inhibited gonadal growth of both genetic sexes, as indicated by the clear dose-dependent decrease in GSI at 270 and 400 dph. The results showed that exposure to E2 during the early life stages of tiger puff er disrupted gonadal development, but that fish recovered after migration to clean seawater. The study suggests the potential use of tiger puff er as a valuable indicator species to evaluate the effects of environmental estrogens on marine fish, thereby protecting valuable fishery resources.
Similar content being viewed by others
Change history
08 September 2017
A mistake was found in Section 2.2. The current expression is “In each treatment group, juveniles were exposed to E2 at the set concentration for 2 h once a day from 15 to 100 dph.” The correct version should be “In each treatment group, juveniles were exposed to E2 at the set concentration for 2 h once two days from 15 to 100 dph.”
References
Allner B, von der Gönna S, Griebeler E M, Nikutowski N, Weltin A, Stahlschmidt-Allner P. 2010. Reproductive functions of wild fish as bioindicators of reproductive toxicants in the aquatic environment. Environmental Science and Pollution Research, 17 (2): 505–518.
Bahamonde P A, Munkittrick K R, Martyniuk C J. 2013. Intersex in teleost fish: are we distinguishing endocrine disruption from natural phenomena? General and Comparative Endocrinology, 192: 25–35.
Balch G C, Mackenzie C A, Metcalfe C D. 2004. Alterations to gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17a-ethinylestradiol. Environmental Toxicology and Chemistry, 23 (3): 782–791.
Baumann L, Holbech H, Keiter S, Kinnberg K L, Knörr S, Nagel T, Braunbeck T. 2013. The maturity index as a tool to facilitate the interpretation of changes in vitellogenin production and sex ratio in the Fish Sexual Development Test. Aquatic Toxicology, 128-129: 34–42.
Bhandari R K, Deem S L, Holliday D K, Jandegian C M, Kassotis C D, Nagel S C, Tillitt D E, vom Saal F S, Rosenfeld C S. 2015. effects of the environmental estrogenic contaminants bisphenol A and 17a-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. General and Comparative Endocrinology, 214: 195–219.
Brion F, Tyler C R, Palazzi X, Laillet B, Porcher J M, Garric J, Flammarion P. 2004. Impacts of 17ß-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile-and adultlife stages in zebrafish (Danio rerio). Aquatic Toxicology, 68 (3): 193–217.
Depiereux S, Liagre M, Danis L, De Meulder B, Depiereux E, Segner H, Kestemont P. 2014. Intersex occurrence in rainbow trout (Oncorhynchus mykiss) male fry chronically exposed to ethynylestradiol. PLoS One, 9 (7): e98531.
Devlin R H, Nagahama Y. 2002. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture, 208 (3-4): 191–364.
Dias L C A, Soares A M V M, Ferreira A L G, Santos C S, Monteiro M S. 2014. Biomarkers of endocrine disruption in juveniles and females of the estuarine fish Pomatoschistus microps. Marine Pollution Bulletin, 84 (1-2): 314–321.
Gorshkov S, Gorshkova G, Colorni B, Gordin H. 2004. Effects of natural estradioI-17ß and synthetic 17a-ethynylestradiol on direct feminization of European Sea Bass Dicentrarchus labrax. Journal of the World Aquaculture Society, 35 (2): 167–177.
Harrison P T C, Holmes P, Humfrey C D N. 1997. Reproductive health in humans and wildlife: are adverse trends associated with environmental chemical exposure? Sci. Total Environ., 205 (2-3): 97–106.
Hattori N, Miyashita S, Sawada Y. 2012. Effective masculinization method of tiger puff er by temperature control under culture condition. Nippon Suisan Gakkaishi, 78 (1): 87.
Hu P, Liu X F, Liu B, Wen H S, Yang Z, Lei J L. 2015. Histological observation on the gonadal differentiation of tiger puff er (Takifugu rubripes). Periodical of Ocean University of China, 45 (10): 25–30. (in Chinese with English abstract)
Jobling S, Beresford N, Nolan M, Rodgers-Gray T, Brighty G C, Sumpter J P, Tyler C R. 2002. Altered sexual maturation and gamete production in Wild Roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biology of Reproduction, 66 (2): 272–281.
Jobling S, Sumpter J P, Sheahan D, Osborne J A, Matthiessen P. 1996. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environmental Toxicology and Chemistry, 15 (2): 194–202.
Kakimoto Y, Aida S, Arai K, Suzuki R. 1994. Induction of gynogenetic diploids in ocellated puff er Takifugu rubripes by cold and heat treatments. J. Fac. Appl. Biol. Sci. Hiroshima Univ., 33 (2): 103–112.
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, Tohari S, Brenner S, Miyadai T, Venkatesh B, Suzuki Y, Kikuchi K. 2012. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger puff erfish, Takifugu rubripes (fugu). PLoS Genetics, 8 (7): e1002798.
Katamachi D, Ikeda M, Uno K. 2015. Identification of spawning sites of the tiger puff er Takifugu rubripes in Nanao Bay, Japan, using DNA analysis. Fisheries Science, 81 (3): 485–494.
Kramer V J, Miles-Richardson S, Pierens S L, Giesy J P. 1998. Reproductive impairment and induction of alkaline-labile phosphate, a biomarker of estrogen exposure, in fathead minnows (Pimephales promelas) exposed to waterborne 17ß-estradiol. Aquatic Toxicology, 40 (4): 335–360.
Länge R, Hutchinson T H, Croudace C P, Siegmund F, Schweinfurth H, Hampe P, Panter G H, Sumpter J P. 2001. effects of the synthetic estrogen 17a-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry, 20 (6): 1216–1227.
Lee K H, Yamaguchi A, Rashid H, Kadomura K, Yasumoto S, Matsuyama M. 2009a. Estradiol-17ß treatment induces intersexual gonadal development in the puff erfish, Takifugu rubripes. Zoological Science, 26 (9): 639–645.
Lee K H, Yamaguchi A, Rashid H, Kadomura K, Yasumoto S, Matsuyama M. 2009b. Germ cell degeneration in hightemperature treated puff erfish, Takifugu rubripes. Sexual Development, 3 (4): 225–232.
Lei B L, Kang J, Yu Y X, Zha J M, Li W, Wang Z J, Wang Y P, Wen Y. 2014. Long-term exposure investigating the estrogenic potency of estriol in Japanese medaka (Oryzias latipes). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 160: 86–92.
Meijide F J, Rey Vázquez G, Piazza Y G, Babay P A, Itria R F, Lo Nostro F L. 2016. Effects of waterborne exposure to 17ß-estradiol and 4-tert-octylphenol on early life stages of the South American cichlid fish Cichlasoma dimerus. Ecotoxicology and Environmental Safety, 124: 82–90.
Mills L J, Chichester C. 2005. Review of evidence: are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Science of the Total Environment, 343 (1-3): 1–34.
Murua H. 2014. Fish reproduction assortment: a wonderful diversity. Environmental Biology of Fishes, 97 (3): 329–333.
Nakamura M. 1984. effects of estradiol-17ß on gonadal sex differentiation in two species of salmonids, the masu salmon, Oncorhynchus Masou, and the chum salmon, O. Keta. Aquaculture, 43 (1-3): 83–90.
Nimrod A C, Benson W H. 1998. Reproduction and development of Japanese medaka following an early life stage exposure to xenoestrogens. Aquatic Toxicology, 44 (1-2): 141–156.
Patyna P J, Davi R A, Parkerton T F, Brown R P, Cooper K R. 1999. A proposed multigeneration protocol for Japanese medaka (Oryzias latipes) to evaluate effects of endocrine disruptors. The Science of the Total Environment, 233 (1-3): 211–220.
Paul-Prasanth B, Shibata Y, Horiguchi R, Nagahama Y. 2011. Exposure to diethylstilbestrol during embryonic and larval stages of medaka fish (Oryzias latipes) leads to sex reversal in genetic males and reduced gonad weight in Genetic Females. Endocrinology, 152 (2): 707–717.
Peters R E M, Courtenay S C, Hewitt L M, MacLatchy D L. 2010. effects of 17a-ethynylestradiol on early-life development, sex differentiation and vitellogenin induction in mummichog (Fundulus heteroclitus). Marine Environmental Research, 69 (3): 178–186.
Piferrer F. 2001. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture, 197 (1-4): 229–281.
Rocha M J, Cruzeiro C, Reis M, Pardal M Â, Rocha E. 2014. Spatial and seasonal distribution of 17 endocrine disruptor compounds in an urban estuary (Mondego River, Portugal): evaluation of the estrogenic load of the area. Environmental Monitoring and Assessment, 186 (6): 3337–3350.
Scholz S, Gutzeit H O. 2000. 17-a-ethinylestradiol aff ects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquat. Toxicol., 50 (4): 363–373.
Segner H, Caroll K, Fenske M, Janssen C R, Maack G, Pascoe D, Schäfers C, Vandenbergh G F, Watts M, Wenzel A. 2003. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project. Ecotoxicology and Environmental Safety, 54 (3): 302–314.
Segner H. 2009. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 149 (2): 187–195.
Seki M, Yokota H, Matsubara H, Tsuruda Y, Maeda M, Tadokoro H, Kobayashi K. 2002. Effect of ethinylestradiol on the reproduction and induction of vitellogenin and testis-ova in medaka (Oryzias Latipes). Environmental Toxicology and Chemistry, 21 (8): 1692–1698.
Shinomiya A, Shibata N, Sakaizumi M, Hamaguchi S. 2002. Sex reversal of genetic females (XX) induced by the transplantation of XY somatic cells in the medaka, Oryzias latipes. Int. J. Dev. Biol., 46 (5): 711–717.
Suzuki N. 1997. Histological study on intersexual gonads observed in wild tiger puff er, takifugu rubripes. Bull. Nansei Natl. Fish. Res. Inst., 30: 101–113.
Van den Belt K, Berckmans P, Vangenechten C, Verheyen R, Witters H. 2004. Comparative study on the in vitro/in vivo estrogenic potencies of 17ß-estradiol, estrone, 17a-ethynylestradiol and nonylphenol. Aquatic Toxicology, 66 (2): 183–195.
Wang H P, Gao Z X, Beres B, Ottobre J, Wallat G, Tiu L, Rapp D, O’Bryant P, Yao H. 2008. effects of estradiol-17ß on survival, growth performance, sex reversal and gonadal structure of bluegill sunfish Lepomis macrochirus. Aquaculture, 285 (1-4): 216–223.
Xu H, Yang J, Wang Y X, Jiang Q, Chen H, Song H Y. 2008. Exposure to 17a-ethynylestradiol impairs reproductive functions of both male and female zebrafish (Danio rerio). Aquatic Toxicology, 88 (1): 1–8.
Zhang X Y, Zha J M, Wang Z J. 2008. Influences of 4-nonylphenol on doublesex-and mab-3-related transcription factor 1 gene expression and vitellogenin mRNA induction of adult rare minnow (Gobiocypris rarus). Environmental Toxicology and Chemistry, 27 (1): 196–205.
Zhao Y B, Wang C, Xia S, Jiang J Q, Hu R, Yuan G X, Hu J Y. 2014. Biosensor medaka for monitoring intersex caused by estrogenic chemicals. Environmental Science & Technology, 48 (4): 2413–2420.
Zheng B H, Liu R Z, Liu Y, Jin F, An L H. 2015. Phenolic endocrine-disrupting chemicals and intersex in wild crucian carp from Hun River, China. Chemosphere, 120: 743–749.
Zillioux E J, Johnson I C, Kiparissis Y, Metcalfe C D, Wheat J V, Ward S G, Liu H. 2001. The sheepshead minnow as an in vivo model for endocrine disruption in marine teleosts: a partial life-cycle test with 17a-ethynylestradiol. Environmental Toxicology and Chemistry, 20 (9): 1968–1978.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the China Agriculture Research System (No. CARS- 50-G20), the National Natural Science Foundation of China (No. 31402284), and the National High Technology Research and Development Program of China (863 Program) (No. 2012AA10A413-2)
An erratum to this article is available at https://doi.org/10.1007/s00343-017-7000-x.
Rights and permissions
About this article
Cite this article
Hu, P., Liu, B., Meng, Z. et al. Recovery of gonadal development in tiger puffer Takifugu rubripes after exposure to 17β-estradiol during early life stages. Chin. J. Ocean. Limnol. 35, 613–623 (2017). https://doi.org/10.1007/s00343-017-6016-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-017-6016-6