Abstract
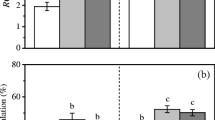
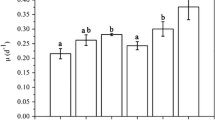
Pyropia haitanensis, a commercially important species, was cultured at two CO2 concentrations (390×10−6 and 700×10−6 (parts per million)) and at low and high nutrient levels, to explore the effect of elevated CO2 on the species under nutrient enrichment. Results show that in CO2-enriched thalli, relative growth rate (RGR) was enhanced under nutrient enrichment. Elevated CO2 decreased phycobiliprotein (PB) contents, but increased the contents of soluble carbohydrates. Nutrient enrichment increased the contents of chlorophyll a (Chl a) and PB, while soluble carbohydrate content decreased. CO2 enrichment enhanced the relative maximum electronic transport rate and light saturation point. In nutrient-enriched thalli the activity of nitrate reductase (NRA) increased under elevated CO2. An instantaneous pH change in seawater (from 8.1 to 9.6) resulted in reduction of NRA, and the thalli grown under both elevated CO2 and nutrient enrichment exhibited less pronounced reduction than in algae grown at the ambient CO2. The thermal optima of NRA under elevated CO2 and/or nutrient enrichment shifted to a lower temperature (10–15°C) compared to that in ambient conditions (20°C). We propose that accelerated photosynthesis could result in growth increment. N assimilation remained high in acidified seawater and reflected increased temperature sensitivity in response to elevated CO2 and eutrophication.
Similar content being viewed by others
References
Alexandre A J, Silva P, Buapet M, Björk R S. 2012. Effects of CO2 enrichment on photosynthesis, growth, and nitrogen metabolism of the seagrass Zostera noltii. Ecology and Evolution, 2: 2 620–2 630.
Andría J R, Brun F G, Pérez-Llorénsand J L, Vergara J J. 2001. Acclimation responses of Gracilaria sp. (Rhodophyta) and Enteromorpha intestinalis (Chlorophyta) to changes in the external inorganic carbon concentration. Bot. Mar., 44: 361–370.
Andría J R, Vergara J J, Perez-Llorens J L. 1999. Biochemical responses and photosynthetic performance of Gracilaria sp. (Rhodophyta) from Cadiz, Spain, cultured under different inorganic carbon and nitrogen levels. Eur. J. Phycol., 34: 497–504.
Axelsson L, Larsson C, Ryberg H. 1999. Affinity, capacity and oxygen sensitivity of two different mechanisms for bicarbonate utilization in Ulva lactuca L. (Chlorophyta). Plant Cell Environ., 22: 969–978.
Beer S, Eshe A. 1985. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust. J. Mar. Fresh., 36: 785–792.
Berges J A, Varela D E, Harrison P J. 2002. Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Mar. Ecol. Prog. Ser., 225: 139–146.
Boulus A, Spaneir E, Friedlander M. 2007. Effect of outdoor conditions on growth rate and chemical composition of Gelidium crinale in culture. J. Appl. Phycol., 19: 471–478.
Brown M B. 2014. The effect of rising ocean temperature and pCO2 on the physiology and growth of giant kelp, Macrocystis pyrifera, and grazing by Purple urchins, Strongylocentrotus purpuratus. http://hdl.handle.net/10211.3/115527.
Chauvin A, Vianne D, Cuet P. 2011. Is the response of coral calcification to seawater acidification related to nutrient loading? Coral Reefs, 30: 911–923.
Christa C, White A. 1999. Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res., 59: 63–72.
Connell S D, Russell B D. 2010. The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests. P. Roy. Soc. B-Biol. Sci., 277: 1 409–1 415.
Corzo A, Niell F X. 1991. Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. Exp. Mar. Biol. Ecol., 146: 181–191.
Diaz-Pulido G, Gouezo M, Tilbrook B, Dove S, Anthony K R. 2011. High CO2 enhances the competitive strength of seaweeds over corals. Ecological Letters, 14: 156–162.
Doney S C, Fabry V J, Feely R A, Kleypas J A. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Ma. Sci., 1: 169–192.
Feely R A, Sabine C L, Lee K, Berelson W, Kleypas J, Fabry V J, Millero F J. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science, 305: 362–366.
Figueroe F L, Conde-Álvarez R, Gómez I. 2003. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth. Res., 75: 259–275.
Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M. 1991. Enhanced growth of the red alga Pyropia yezoensis Ueda in high CO2 concentrations. J. Appl. Phycol., 3: 356–362.
Gao Y, Smith G J, Alberte R S. 2000. Temperature dependence of nitrate reductase activity in marine phytoplankton: biochemical analysis and ecological implications. J. Phycol., 36: 304–313.
Gardner W S, Wynne D S, Dunstan W M. 1976. Simplified procedure for the manual analysis of nitrate in seawater. Mar. Chem., 4: 393–396.
Gordillo F J L, Figueroa F L, Niell F X. 2003. Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta, 218: 315–322.
Gordillo F J L, Niell F X, Figueroa F T. 2001. Nonphotosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta., 213: 64–70.
Gutow L, Rahman M M, Bartl K, Saborowski R, Bartsch I, Wiencke C. 2014. Ocean acidification affects growth but not nutritional quality of the seaweed Fucus vesiculosus (Phaeophyceae, Fucales). J. Exp. Mar. Biol. Ecol., 453: 84–90.
Harrison P J. 1988. Determining phosphate uptake rates of phytoplankton. In: Lobban C S ed. Experimental Phycology: A Laboratory Manual. New York Cambridge Univ. Press. p.186–195.
Hofmann L C, Yildiz G, Hanelt D, Bischof K. 2012. Physiological responses of the calcifying rhodophyte Corallina officinalis (L.) to future CO2 levels. Mar. Biol., 159: 783–792.
Huppe H C, Turpin D H. 1994. Integration of carbon and nitrogen metabolism in plant and algal cells. Annu. Rev. Plant Biol., 45: 577–607.
Israel A, Hophy M. 2002. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentration. Glob. Chang. Biol., 8: 831–840.
Jassby A T, Platt T. 1976. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Oceanography, 21: 540–547.
Jimenez del R, Ramazanov M, Reina Z G G. 1995. Effect of nitrogen supply on photosynthesis and carbonic anhydrase activity in the green seaweeds Ulva rigida (Chlorophyta). Mar. Biol., 123: 687–691.
Kennison R L, Fong P. 2013. Extreme eutrophication in shallow estuaries and lagoons of California is driven by a unique combination of local watershed modifications that trump variability associated with wet and dry seasons. Estuaries and Coasts, http://dx.doi.org/10.1007/s12237-013-9687-z.
Koch M, Bowes G, Ross C, Zhang X H. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global. Change. Biol., 19: 103–132.
Kübler J E, Johnston A M, Raven J A. 1999. The effects reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant Cell Environ., 22: 1 303–1 310.
Lapointe B E, Duke C S. 1984. Biochemical strategies for growth of Gracilaria tikuahiae (Rhodophyta) in relation to light intensity and nitrogen availability. J. Phycol., 20: 488–495.
Larsson C M, Larsson M. 1987. Regulation of nitrate utilization in green algae. In: Ullrich W W ed. Inorganic Nitrogen Metabolism. Springer-Verlag, New York. p.203–207.
Liu D Y, Keesing J K, He P M, Wang Z L, Shi Y J, Wang Y J. 2013. The world’s largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuarine, Coastal and Shelf Science, 129: 2–10.
Ma Z, Gao K. 2010. Spiral breakage and photo-inhibition of Arthrospira platensis (Cyanophyta) caused by accumulation of reactive oxygen species under solar radiation. Environ. Exp. Bot., 68: 208–213.
Martin S, Rodolfo-Metalpa R, Ransome E, Rowley S, Buia M C, Gattuso J P, Hall-Spencer J. 2008. Effects of naturally acidified seawater on seagrass calcareous epibionts. Biological Letters, 4: 689–692.
Olischläger M, Wiencke C. 2013. Ocean acidification alleviates low-temperature effects on growth and photosynthesis of the red alga Neosiphonia harveyi (Rhodophyta). J. Exp. Bot., 18: 5 587–5 597.
Orr J C, Fabry V J, Aumont O et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437: 681–686.
Parry M A, Andralojc P J, Scales J C, Salvucc M E, Carmo-SilvaA E, Alonso H, Whitney S M. 2013. Rubisco activity and regulation as targets for crop improvement. J. Exp. Bot., 64: 717–730.
Raven J A, Giordano M, Beardall J, Maberly S C. 2012. Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philosophical Transactions of the Royal Society B: Biol. Sci., 367: 493–507.
Sabine C L, Feely R A. 2007. The oceanic sink for carbon dioxide. In: Reay D et al ed. Greenhouse Gas Sinks. CABI Publishing, Oxfordshire. p.31–39.
Sabine G L, Feely R A, Gruber N et al. 2004. The oceanic sink for anthropogenic CO2. Science, 305: 367–371.
Sarker M Y, Bartsch I, Olischläger M, Gutow L, Wiencke C. 2013. Combined effects of CO2, temperature, irradiance and time on the physiological performance of Chondrus crispus (Rhodophyta). Bot. Mar., 56: 63–74.
Schreiber U, Hormann H, Neubauer C, Klughammer C. 1995. Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Funct. Plant. Biol., 22: 209–220.
Sheen J. 1994. Feedback control of gene expression. Photosynth. Res., 39: 427–438.
Suárez-Álvarez S, Gómez-Pinchetti J L, García-Reina G. 2012. Effect of increased CO2 levels on growth, photosynthesis, ammonium uptake and cell composition in the macroalga Hypnea spinella (Gigartinales, Rhodophyta). J. Appl. Phycol., 24: 815–823.
Turpin D. 1991. Effect of inorganic availability on algal photosynthesis and carbon metabolism. J. Phycol., 27: 14–20.
Wellbum A R. 1994. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Plant Physiol., 144: 307–313.
Young E, Dring M J, Savidge G, Birkett D A, Berges J. 2007. Seasonal variations in nitrate reductase activity and internal N pools in intertidal brown algae are correlated with ambient nitrate concentrations. Plant Cell Environ., 30: 764–774.
Yu G. 2000. Temperature dependence of nitrate reductase activity in marine phytoplankton: biochemical analysis and ecological implications. J. Phycol., 36: 304–313.
Zou D H. 2005. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture, 250: 726–735.
Zou D H, Gao K S. 2002. Photosynthetic responses to inorganic carbon in Ulva lactuca under aquatic and aerial states. Acta Bot. Sin., 44: 1 291–1 296.
Zou D H, Gao K S, Xia J R. 2003. Photosynthetic utilization of inorganic carbon in the economic brown alga, Hizikia fusiforme (Sargassaceae) from the South China Sea. J. Phycol., 39: 1 095–1 100.
Zou D H, Gao K S. 2009. Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia, 48: 510–517.
Zou D H, Gao K S, Luo H J. 2011. Short- and long-term effects of elevated CO2 on photosynthesis and respiration in the marine macroalgae Hizikia Fusiformis (Sargassaceae, Phaeophyta) grown at low and high N supplies. J. Phycol., 47: 87–97.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Nos. 41276148, 41076094)
Rights and permissions
About this article
Cite this article
Liu, C., Zou, D. Effects of elevated CO2 on the photosynthesis and nitrate reductase activity of Pyropia haitanensis (Bangiales, Rhodophyta) grown at different nutrient levels. Chin. J. Ocean. Limnol. 33, 419–429 (2015). https://doi.org/10.1007/s00343-015-4057-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-015-4057-2