Abstract
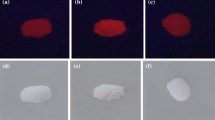
Structural, optical and photoluminescent properties of La2−xPrx(MoO4)3 phosphors with different doping concentrations of Pr3+, synthesized by conventional solid-state and solution combustion routes were studied. X-ray diffraction studies of compounds synthesized through conventional solid-state ceramic route confirm that all samples crystallized in monoclinic La2(MoO4)3 structure with c2/c space group, while combustion synthesized compounds show the existence of two different crystal environments. Analysis of UV–visible diffuse reflectance spectra (DRS) shows characteristics of absorption bands in blue and red regions for Pr3+ substituted La2(MoO4)3 samples, synthesized by both methods. The calculated band gap from the DR spectrum showed an inverse dependence with Pr3+ doping concentration for solid-state synthesized La2−xPrx(MoO4)3 samples, while for combustion synthesized La2−xPrx(MoO4)3 compounds the variation of band gap with concentration is not monotonous. The photoluminescence emission spectrum of blue excited La2−xPrx(MoO4)3 phosphors synthesized by both routes showed similar multicoloured emission bands. Maximum emission intensity was observed for Pr3+ concentration of x = 0.05 in both synthesis methods. The exchange interaction between nearest activator ions leads to concentration quenching of luminescence for solid-state synthesized La2−xPrx(MoO4)3 compounds and dipole–dipole interaction for combustion synthesized La2−xPrx(MoO4)3 compounds. The average emission colour of the blue excited La2−xPrx(MoO4)3 phosphors lies in the yellow–orange region. The CCT values lie in the warm light region and the luminescence lifetime of 8 µs for red emission peak showed promising applications requiring fast switching response.














Similar content being viewed by others
Data availability
Data will be made available on request.
References
J. McKittrick, L.E. Shea-Rohwer, Review: down conversion materials for solid-state lighting. J. Am. Ceram. Soc. 97(5), 1327–1352 (2014). https://doi.org/10.1111/jace.12943
M.-H. Fang, J.L. Leaño, R.-S. Liu, Control of narrow-band emission in phosphor materials for application in light-emitting diodes. ACS Energy Lett. 3(10), 2573–2586 (2018). https://doi.org/10.1021/acsenergylett.8b01408
L. Wang, R.-J. Xie, T. Suehiro, T. Takeda, N. Hirosaki, Down-conversion nitride materials for solid state lighting: recent advances and perspectives. Chem. Rev. 118(4), 1951–2009 (2018). https://doi.org/10.1021/acs.chemrev.7b00284
S. Nakamura, T. Mukai, M. Senoh, Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 64(13), 1687–1689 (1994). https://doi.org/10.1063/1.111832
C.C. Lin, R.S. Liu, Y.S. Tang, S.F. Hu, Full-color and thermally stable KSrPO4: Ln (Ln=Eu, Tb, Sm) phosphors for white-light-emitting diodes. J. Electrochem. Soc. 155(9), J248–J251 (2008). https://doi.org/10.1149/1.2953591
T. Nishida, T. Ban, N. Kobayashi, High-color-rendering light sources consisting of a 350-nm ultraviolet light-emitting diode and three-basal-color phosphors. Appl. Phys. Lett. 82(22), 3817–3819 (2003). https://doi.org/10.1063/1.1580649
M.-H. Fang, Z. Bao, W.-T. Huang, R.-S. Liu, Evolutionary generation of phosphor materials and their progress in future applications for light-emitting diodes. Chem. Rev. 122(13), 11474–11513 (2022). https://doi.org/10.1021/acs.chemrev.1c00952
X. Piao, K. Machida, T. Horikawa, H. Hanzawa, Self-propagating high temperature synthesis of yellow-emitting Ba2Si5N8:Eu2+ phosphors for white light-emitting diodes. Appl. Phys. Lett. 91(4), 041908-1-041908–3 (2007). https://doi.org/10.1063/1.2760038
L.Đ Far, T. Dramićanin, M. Medić, Z. Ristić, J. Periša, V. Đorđević, Ž Antić, M.D. Dramićanin, Emission color tunability of dysprosium-activated YNbO4–LuNbO4-mixed phosphors. Appl. Phys. A 130(2), 107 (2024). https://doi.org/10.1007/s00339-023-07271-z
C.C. Lin, R.-S. Liu, Advances in phosphors for light-emitting diodes. J. Phys. Chem. Lett. 2(11), 1268–1277 (2011). https://doi.org/10.1021/jz2002452
B. Xu, J. Liu, C. Song, H. Luo, Y. Jie Peng, X. Yu, Synthesis and tunable luminescent properties of red phosphor Li1−mAgmLa0.99−nYnPr0.01(MoO4)2 with blue excitation for white LEDs. J. Am. Ceram. Soc. 95(1), 250–256 (2012). https://doi.org/10.1111/j.1551-2916.2011.04764.x
Y. Liang, F. Liu, Y. Chen, X. Wang, K. Sun, Z. Pan, Red/near-infrared/short-wave infrared multi-band persistent luminescence in Pr3+-doped persistent phosphors. Dalton Trans. 46, 11149–11153 (2017). https://doi.org/10.1039/C7DT02271A
I. Mackeviciute, A. Linkeviciute, A. Katelnikovas, Synthesis and optical properties of Y2Mo4O15 doped by Pr3+. J. Lumin. 190, 525–530 (2017). https://doi.org/10.1016/j.jlumin.2017.06.014
A.M. Srivastava, Inter- and intraconfigurational optical transitions of the Pr3+ ion for application in lighting and scintillator technologies. J. Lumin. 129(12), 1419–1421 (2009). https://doi.org/10.1016/j.jlumin.2009.01.041
J. Grigorjevaite, A. Katelnikovas, Synthesis and optical properties investigation of blue-excitable red-emitting K2Bi(PO4) (MoO4):Pr3+ powders. J. Mater. Res. Technol. 9(6), 15779–15787 (2020). https://doi.org/10.1016/j.jmrt.2020.11.054
H. Lee, W.J. Chung, W. BinIm, Pr3+-doped oxyfluoride glass ceramic as a white LED color converter wide color gamut. J. Lumin. 236, 118064 (2021). https://doi.org/10.1016/j.jlumin.2021.118064
T. Kyômen, R. Sakamoto, N. Sakamoto, S. Kunugi, M. Itoh, Photoluminescence properties of Pr-doped (Ca, Sr, Ba)TiO3. Chem. Mater. 17(12), 3200–3204 (2005). https://doi.org/10.1021/cm0403715
P. Boutinaud, E. Pinel, M. Oubaha, R. Mahiou, E. Cavalli, M. Bettinelli, Making red emitting phosphors with Pr3+. Opt. Mater. 28(1), 9–13 (2006). https://doi.org/10.1016/j.optmat.2004.09.027
G.B. Nair, H.C. Swart, S.J. Dhoble, A review on the advancements in phosphor-converted light emitting diodes (pc-LEDs): phosphor synthesis, device fabrication and characterization. Prog. Mater. Sci. 109, 100622 (2020). https://doi.org/10.1016/j.pmatsci.2019.100622
G. Benoît, J. Véronique, A. Arnaud, G. Alain, Luminescence properties of tungstate’s and molybdates phosphors: illustration on ALn(MO4)2 compounds (A = alkaline cation, Ln = lanthanides, M = W, Mo). Solid State Sci. 13(2), 460–467 (2011). https://doi.org/10.1016/j.solidstatesciences.2010.12.013
Y. Tian, X. Qi, X. Wu, R. Hua, B. Chen, Luminescent properties of Y2(MoO4)3: Eu3+ red phosphors with flowerlike shape prepared via coprecipitation method. J. Phys. Chem. C 113(24), 10767–10772 (2009). https://doi.org/10.1021/jp901053q.fo
R. Satheesh, M. Venugopal, H.P. Kumar, Influence of structural variation on the optical properties of Y2−xSmxMo3O12 phosphors. J. Mater. Sci. Mater. Electron. 33, 16837–16855 (2022). https://doi.org/10.1007/s10854-022-08554-6
R. Satheesh, Meenu Venugopal, S.P. Anusree, V.S. Dhanya, H. PadmaKumar, Optical characterization of rare-earth activated La2−xLnx (MoO4)3 (Ln=Dy, Sm) phosphors. J. Mol. Struct. 1281, 135111 (2023). https://doi.org/10.1016/j.molstruc.2023.135111
N. Huang, G. Lu, B. Bai, H. Zhao, W. Yao, C. Cao, Y. Li, A. Xie, Preparation, crystal structure, and photoluminescence properties of Tb3+ activated Lu2(MoO4)3 green-emitting phosphors. J. Lumin. 269, 120475 (2024). https://doi.org/10.1016/j.jlumin.2024.120475
M. Tsvetkov, D. Elenkova, M. Milanova, Luminescence properties of Gd2(MoO4)3 modified with Sm(III) and Tb(III) for potential LED applications. Crystals (Basel) 12(1), 120 (2022). https://doi.org/10.3390/cryst12010120
E. Pinel, P. Boutinaud, R. Mahiou, Using a structural criterion for the selection of red-emitting oxide-based compounds containing Pr3+. J. Alloys Compd. 374(1–2), 165–168 (2004). https://doi.org/10.1016/j.jallcom.2003.11.084
Y. Tian, B. Chen, R. Hua, J. Sun, L. Cheng, H. Zhong, X. Li, J. Zhang, Y. Zheng, T. Yu, L. Huang, H. Yu, Optical transition, electron-phonon coupling and fluorescent quenching of La2(MoO4)3:Eu3+ phosphor. J. Appl. Phys. 109(5), 053511 (2011). https://doi.org/10.1063/1.3551584
C. Guo, T. Chen, L. Luan, W. Zhang, D. Huang, Luminescent properties of R2(MoO4)3:Eu3+ (R=La, Y, Gd) phosphors prepared by sol–gel process. J. Phys. Chem. Solids 69(8), 1905–1911 (2008). https://doi.org/10.1016/j.jpcs.2008.01.021
D. Marrero-López, P. Núñez, M. Abril, V. Lavín, U.R. Rodríguez-Mendoza, V.D. Rodríguez, Synthesis, electrical properties, and optical characterization of Eu3+-doped La2Mo2O9 nanocrystalline phosphors. J. Non-Cryst. Solids 345–346, 377–381 (2004). https://doi.org/10.1016/j.jnoncrysol.2004.08.047
M.-G. Ma, K. Yao, F. Deng, Hydrothermal synthesis and characterization of hierarchically nanostructured La2(MoO4)3. Mater. Lett. 121, 70–73 (2014). https://doi.org/10.1016/j.matlet.2014.01.131
M.L. Myrick, M.N. Simcock, M. Baranowski, H. Brooke, S.L. Morgan, J.N. McCutcheon, The Kubelka–Munk diffuse reflectance formula revisited. Appl. Spectrosc. Rev. 46(2), 140–165 (2011). https://doi.org/10.1080/05704928.2010.537004
A.M. Pires, M.R. Davolos, Luminescence of europium(III) and manganese(II) in barium and zinc orthosilicate. Chem. Mater. 13(1), 21–27 (2001). https://doi.org/10.1021/cm000063g
T. Wang, B. Gao, J. Li, Z. Wang, P. Li, Achieving luminescence of Sr3Ga1.98In0.02Ge4O14:0.03Cr3+ via [In3+] substitution [Ga3+] and its application to NIR pc-LED in non-destructive testing. Molecules 28(24), 8059 (2023). https://doi.org/10.3390/molecules28248059
R. Stankeviciute, A. Zalga, Sol–gel synthesis, crystal structure, surface morphology, and optical properties of Eu2O3-doped La2Mo3O12 ceramic. J. Therm. Anal. Calorim. 118, 925–935 (2014). https://doi.org/10.1007/s10973-014-3882-4
W.T. Carnall, P.R. Fields, K. Rajnak, Electronic energy levels in the trivalent lanthanide aquo ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 49(10), 4424–4442 (1968). https://doi.org/10.1063/1.1669893
J. Tauc, R. Grigorovici, A. Vancu, Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi (b) 15(2), 627–637 (1966). https://doi.org/10.1002/pssb.19660150224
M. Venugopal, H.P. Kumar, R. Satheesh, R. Jayakrishnan, Enhanced photoluminescence in CaZr0.9SmxDy(0.1−x)O3 perovskites by Mg2+ and Al3+ co-doping for WLED applications. Mater. Res. Express 6, 076201 (2019). https://doi.org/10.1088/2053-1591/ab0dbd
E. Burstein, Anomalous optical absorption limit in InSb. Phys. Rev. 93(3), 632–633 (1954). https://doi.org/10.1103/PhysRev.93.632
H. Dornauf, J. Heber, Concentration-dependent fluorescence-quenching in La1−xPrxP5O14. J. Lumin. 22(1), 1–16 (1980). https://doi.org/10.1016/0022-2313(80)90040-X
M. Chen, Study on the structure and luminescence quenching of Pr doped Na0.5Bi4.5Ti4O15 multifunctional ceramics. J. Mater. Sci. Mater. Electron. 30, 20393–20399 (2019). https://doi.org/10.1007/s10854-019-02348-z
J. Stefanska, L. Marciniak, Single-band ratiometric luminescent thermometry using Pr3+ ions emitting in yellow and red spectral ranges. Adv. Photonics Res. 2(7), 2100070 (2021). https://doi.org/10.1002/adpr.202100070
D.L. Dexter, A theory of sensitized luminescence in solids. J. Chem. Phys. 21, 836–850 (1953). https://doi.org/10.1063/1.1699044
C.S. McCamy, Correlated color temperature as an explicit function of chromaticity coordinates. Color Res. Appl. 17(2), 142–144 (1992). https://doi.org/10.1002/col.5080170211
P. Dang, G. Zhang, W. Yang, H. Lian, G. Li, J. Lin, Red–NIR luminescence in rare-earth and manganese ions Codoped Cs4CdBi2Cl12 vacancy-ordered quadruple perovskites. Chem. Mater. 35(4), 1640–1650 (2023). https://doi.org/10.1021/acs.chemmater.2c03246
Acknowledgements
The authors would acknowledge Mar Ivanios College; Nalanchira, Thiruvananthapuram IISER; Trivandrum, STIC; Cusat and CLIFF; University of Kerala, Karivattom for providing analysis facilities.
Author information
Authors and Affiliations
Contributions
Satheesh R: methodology, formal analysis, investigation, software, writing original draft. Anusree S. P: formal analysis, investigation; Dhanya V. S: formal analysis, investigation; H. Padma Kumar: conceptualization, validation, resources, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satheesh, R., Anusree, S.P., Dhanya, V.S. et al. Effect of synthesis method on the structural and optical properties of blue-excitable La2−xPrx(MoO4)3 phosphors. Appl. Phys. A 130, 270 (2024). https://doi.org/10.1007/s00339-024-07449-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07449-z