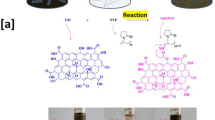
Abstract
Radiation safety and protection is a subject of major concern for all communities because of the biological damage caused by different radiation sources. Therefore, the purpose of this study is aimed to synthesize two different thin films from a newly synthesized N-Al2O3@Pb(II)-2,4-DiNA nanocomposite which was prepared by the direct chemical coupling of nano-aluminum oxide (N-Al2O3) with Pb(II)-2,4-dinitroaniline complex. The synthesized materials were characterized by FT-IR, thermogravimetric analysis (TGA), scanning electron microscope SEM, and elemental composition by energy-dispersive X-ray (EDX). The two thin films were assembled by the encapsulation of N-Al2O3@Pb(II)-2,4-DiNAnanocompositeinto hydrolyzed polyurethane and hydrolyzed polyvinyl chloride. Electron spin resonance (ESR) measurements have been used to investigate the newly prepared nanocomposite and thin films before and after gamma irradiation. Gamma spectroscopy, Phy-X software, and SRIM (stopping and range of ions in matter) Monte Carlo simulation code are aimed to apply and investigate radiation shielding efficiency of films for gamma/x-ray, protons, alpha, and thermal neutrons. The gamma shielding characteristics, mass stopping power (MSP), and range for both proton (H-ions) and alpha (He-ions) were also calculated. In addition, the SRIM code’s subroutine TRIM was used to predict the damage production and atomic displacements per atom (dpa) caused by the interaction of high-energy H-ions (proton) and He-ions (alpha) with the studied samples. The data obtained the good radiation shielding effect of the prepared thin films and their possible use as radiation shielding materials for X-rays and low gamma energy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, ionizing radiation has rapidly been used for many purposes, including medical, scientific, and industrial applications, particularly in the medical field, where X-ray equipment exposes medical staff and crew to direct or scattered X-ray beams [1]. Moreover, treatments using charged particles such as protons and alpha have recently regained interest in the modern radiology field [2, 3]. Shielding materials are therefore becoming increasingly important to protect all radiation workers' employees [3].
Much research is being done to create new combinations of shielding materials, and to investigate the effects of exposure to gamma rays and charged particles and the damage they cause to materials [4].
Diverse publications have reported nanolead oxide and its nanocomposite materials and alumina as effective materials in gamma radiation shielding and sensing [5,6,7,8].
For example, the addition of hematite Fe2O3 to aluminum lead borate glasses for the formation of 55B2O3-30Pb3O4-(15-x) Al2O3-xFe2O3 was previously investigated and reported in gamma radiation shielding [5]. The addition of lead nanoparticles with epoxy/glass fiber composites to obtain fiber-reinforced polymer composites was studied for gamma radiation shielding [6]. Alumina was also investigated in a number of radiation shielding publications and accepted as a shielding material due to its ability to increase the mechanical properties of glass shielding materials [7, 8]. The additional effect of diverse contents of MoO3 on the synthesized B2O3-Pb3O4-Al2O3 glasses was investigated to improve mechanical, and radiation shielding properties of B2O3-Pb3O4-Al2O3 glasses [7]. Another study was reported using micro Al2O3, nanoAl2O3 and Al2O3/TiO2 towards gamma-radiation shielding properties and the collected results indicated that the gamma-shielding properties were increased by the addition of TiO2 [8]. The gamma attenuation was enhanced using high-density polyethylene reinforced with diverse kinds of nanometal oxides like Al2O3, PbO, and Fe2O3 [9].
In the current study, two thin films were assembled from N-Al2O3@Pb(II)-2,4-DiNA nanocomposite and encapsulated into hydrolyzed polyurethane and hydrolyzed polyvinyl chloride matrices and then tested to evaluate their gamma-ray shielding qualities, proton and positron mass stopping powers, and projected ranges (PR).
2 Experimental
2.1 Materials and instrumentations
In this study, all the chemicals were purchased and used without purification and the specifications of all applied chemicals are illustrated in Table 1, while the specifications of various instrumentations are compiled in Table 2. The EDX analysis of the synthesized Pb (II)-2,4-DINA is illustrated in Table 3. On the other hand for the two prepared thin films the EDX chemical analysis data (wt%) are tabulated in Table 4. The two assembled thin films were chemically analyzed by using an electron-dispersive X-ray type (EDAX) FET instrument. Electron spin resonance (ESR) spectra were confirmed on two small samples taken from the films, inserted in ESR tubes and measured at the mentioned instrument parameters of ESR listed in Table 2, before and after exposure to 16 kGy of gamma irradiation using a 60Co gamma cell specified in Table 2.
2.2 Synthesis of Pb(II)-2,4-dinitroaniline complex
A 4.0 g of 2,4-dinitroaniline was dispersed in 20.0 mL of nitrobenzene under vigorous stirring and heating at 120 °C to form a homogenous mixture. A 1.0 g of lead acetate was dissolved in 20.0 mL distilled water and added to the previous mixture under vigorous stirring and heating for 90 min at 90.0 °C. After the stirring two aqueous and organic phases were obtained. This mixture was filtered and a yellow precipitate of Pb(II)-2,4-dinitroaniline complex(Pb(II)-2,6-DiNA)was obtained.
2.3 Synthesis of nano-Al2O3
Nano-Al2O3 was synthesized according to a combustion reaction between Al2(CO3)3 and glycine in a 1:1 ratio (w/w). The two components were mixed very well at room temperature and heated in a muffle furnace at 700 °C for 5 h to produce a white precipitate of Nano-Al2O3 (N-Al2O3).
2.4 Synthesis of nano-Al2O3-linked-Pb(II)-2,4-dinitroaniline nanocomposite
N-Al2O3 and Pb(II)-2,4-DiNA were reacted and combined together by using the formaldehyde as a crosslinker between the two reacting materials for the formation of N-Al2O3@Pb(II)-2,4-DiNA through hydrogen bonding between the hydroxy groups of Alumina and the -NH2 groups of the 2,4 di nitro aniline as illustrated through the FT-IR spectrum according to the following procedure; 2.0 g of N-Al2O3was mixed with 2.0 g of Pb(II)-2,4-dinitroaniline in the presence of 20.0 mL of formaldehyde after that the mixture was refluxed for 6.0 h at 90.0 °C, and the resultant yellowish white powder material was washed several times to remove the excess of the formaldehyde and, it was dried at 60.0 °C for 8.0 h.
2.5 Fabrication of thin films
The prepared (N-Al2O3@Pb(II)-2,4-DiNA) nanocomposite was used to assemble two thin films via the preparation of two different mixtures according to the following procedure; the first mixture was prepared by mixing a stock solution containing 3.0 g (N-Al2O3@Pb(II)-2,4-DiNA) with 30.0 mL of methylene chloride and 5.0 g of completely hydrolyzed polyurethane solution (99–100%) already dissolved in 150.0 mL of double- distilled water at 50.0 °C. The second mixture was prepared by the addition of a stock solution containing 2.0 g (N-Al2O3@Pb(II)-2,6-DiNA) and 30.0 mL dimethylformamide (DMF) to 1.0 g of completely hydrolyzed polyvinyl chloride (PVC) solution (99–100%) already dissolved in 150.0 mL of double-distilled water at 50.0 °C.The above two mixtures were stirred well and poured onto a 10 × 10 cm horizontal glass plate, and left to dry at room temperature for 48 h. The stripped-off thin films were found to have a thickness corresponding to 0.045 ± 0.003 mm as illustrated in Fig. 1.
2.6 Density measurements:
Density measurements were performed according to the Archimedes principle, with xylene as the immersion liquid, and a digital high-sensitivity balance. Density ρ (g/cm3) is calculated from the following relationship [21].
where (Wair), (WLiquid) are the weights of samples in air and xylene samples, respectively, and ρliquid is the density of xylene liquid.
2.7 Shielding parameters
An energetic ion losses energy by inelastic energy transfer to electrons via excitation and ionizing of target atoms and the ion itself, elastic energy transfers to atomic nuclei (recoils), and high-energy effects (such as nuclear reactions and bremsstrahlung) [10, 11].
The rate of energy loss per unit route length (dE/dx) is measured by stopping power, also known as stopping force [12]. Simply, ion energy loss is classified into two types: (1) energy transfer to target electrons (electronic stopping power, dE/dx), which results in ionization, and (2) energy transfer to target nuclei (nuclear-stopping power, dE/dx), which results in atomic displacements or phonon energy dissipation for energy transfers above or below the threshold displacement energy (Ed). Atoms are only displaced if their kinetic energy is greater than their displacement energy [13, 14].
The total stopping power for charged particle interactions is split into two parts, collision stopping power, and radiative stopping power according to Eq. (1).
A Monte Carlo Simulation computer model named Stopping and Range of Ions in Materials, or SRIM (formerly TRIM) is frequently used to calculate electronic and nuclear stopping powers and simulate the inelastic and elastic energy transfers from an energetic incident ion to target atoms. The resulting displacement generation profile is used to quantify the local damage dosage in atomic displacements per atom and to analyze the effects of energetically charged particles on target composition, structure functionality, and surface topography [15].
Linear attenuation coefficient (LAC) values for all samples were obtained using a gamma spectrometer equipped with a 'NaI(Tl) scintillation detector'(Canberra model), a3′ × 3′ amplifier, and a16k multi-channel analyzer. Spectra acquired by multichannel analyzer were analyzed using Genie software.
The radioactive point sources, Cs-137 and Co-60, with gamma energies 662, 1173 and 1333KeVwasinfrontofthesample.The transmitted gamma ray intensity was measured with the sample placed between the radioactive sources and the detector, and the same measurement was performed without the sample between the sources and detector (I0) to assess back ground radiation. Additionally, Phy-X/PSD software is used to determine the gamma attenuation parameter for samples over a wide range of photon energies. This software can calculate the mass attenuation coefficients (MAC), effective atomic number (Zeff) and effective conductivity (Ceff) of fast neutrons [12]. and the effective removal cross-section (ƩR) of fast neutrons. The SRIM Monte Carlo simulation code and its subroutine TRIM were used to calculate the mass stopping power (MSP) and projected range (PR) for protons (H ions) and alpha (He ions) in two samples. In addition, total displaced atoms and damage predictions were calculated. Relevant equations for all measured and estimated shielding parameters were assumed from Refs. [16,17,18] and all depended on descriptions in the Phy-X/PSD software program [19, 20].
The associated equations, as well as the explicit routine interpretations for gamma shielding parameters such as LAC, MAC, Ceff, RPE %, the macroscopic cross-section for absorption (Ʃa), coherent scattering (Ʃco), and incoherent scattering (Ʃinco) of thermal neutrons (at energy = 0.025 eV); effective removal cross-sections for fast neutrons (ƩR), mass stopping power (MSP) with ranges were assumed from references [16,17,18], all of them were dependent upon the descriptions of Phy-X/PSD software program [19, 20].
3 Results and discussion
3.1 FT-IR characterization
The synthesized materials in this work were characterized by various techniques like FT-IR, TGA, EDX, and SEM to investigate and confirm the successful synthesis of a newly assembled nanocomposite (N-Al2O3@Pb(II)-2,4-DiNA). Figure 2a illustrates the presence of Al2O3 by the presence of two characteristic peaks at 473.72, and 1101.80 cm−1 due to Al-O bonding, the two other peaks at 1643.10 and 3392.36 cm−1 are ascribed to the adsorbed water molecule on the N-Al2O3 surface [22]. The FT-IR spectrum of Pb(II)-2,4-DiNA is illustrated in Fig. 2b and shows the peaks at 472.77, 512.26, and 596.26 cm−1 which are mainly related to the Pb–O bonding. The peaks at 730.22, 806.46, 853.67, and 880.52 are ascribed to the stretching vibration of C–N in (–NO2) group [23, 24]. The band at 1243.70 cm−1 is assigned to the -NH2 group. The peaks of 2, 4-dinitroaniline are illustrated by the two bands at 1356.39 and 1558.43 cm−1 which are attributed to the –NO2 group. The absorption peak at 1356.39 cm−1 is due to N–O symmetric stretching due to the presence of nitro compounds. A band near 1425.71 cm−1 can be attributed to a strong metal–oxygen bond in Pb–O of the nitro group in the Pb(II)-2,4-DiNA [23, 24] according to Scheme 1. The peaks at 1511.87, and 1629.57 cm−1 are related to –C=C bond in the benzene ring. The peak at 3096.54 cm−1 is due to C–H stretching, while the absorption peak at 3335.40 cm−1 refers to N–H stretching due to the presence of the amine group. The broad band at 3453.72 cm−1 is mainly due to the absorption of water moisture by Pb(II)-2,4-DiNA nanocomposite. Finally, Fig. 2c provides good evidence for the successful preparation of (N-Al2O3@Pb(II)-2,4-DiNA) nanocomposite with a small shift and a modification in the peak positions. It was observed that, the area around the peak at 501.53 cm−1 is mainly related to the metal oxygen bond Pb–O, or Al-O bonding. The small peak at 880.38 cm−1 instead of the three peaks of 805.46, 853.46, and 880.52 due to the stretching vibration of C–N in (–NO2) group [24, 25]. The peaks of 2, 4-dinitroaniline are illustrated by the two bands at 1357.63 and 1560.30 cm−1 which are attributed to the –NO2 group, while the absorption peak at 1357.63 cm−1 is due to N–O symmetric stretching due to the presence of nitro compounds. The band at 1261.14 cm−1 is assigned to the –NH2 group, a band near 1432.34 cm−1 can be attributed to a strong metal–oxygen bond in Pb–O of the nitro group in the Pb(II)-2,4-DiNA.The peak at 1632.12 cm−1 is related to –C=C bond in the benzene ring. The absorption band at 3338.89 cm−1 is due to the N–H stretching in the amine group. The peak at 3096.54 cm−1 is due to C–H stretching, while the absorption peak at 3455.63 cm−1 is referring to N–H stretching due to the presence of the amine group. The broad band at 3455.63 cm−1 is mainly due to the absorption of water moisture by Pb(II)-2,4-DiNA nanocomposite [24, 25].
3.2 TGA characterization
The TGA analysis for the three synthesized materials (N-Al2O3, Pb(II)-2,4-DiNA and N-Al2O3@Pb(II)-2,4-DiNA) is represented in Fig. 3. Figure 3a illustrates the thermogram and thermal degradation of N-Al2O3 based on three steps. The first step (at 23.98–103.69 °C) refers to 3.28% degradation, while the second degradation step (at 103.69–297.57 °C) to confirm a 3.12% loss. The degradation in these two steps is mainly due the loss of moisture content from the metal oxide. The third step (at 297–598 °C) may be caused by the loss of water of crystallization from N-Al2O3. Figure 3b shows the thermogram of Pb(II)-2,4-DiNA which illustrates the following degradation steps: the first step (at 45.09–153.82 °C) refers to 2.35% degradation due to moisture content, the second step (at 153.82–232.69 °C) shows 60.56% loss due to the degradation of 2,4-dinitroaniline moiety via sharp degradation and cracking into its main constituent groups as amino, nitro and benzene rings. Moreover, the thermogram refers to 35% stability of the total sample weight to indicate the presence of lead in Pb(II)-2,4-DiNA. Finally, Fig. 3c represents the thermogram of N-Al2O3@Pb(II)-2,4-DiNA which is characterized by the presence of four decomposition steps. The first step (at 29.13–61.29 °C with 3.29% loss), the second step (at 61.29–157.21 °C with 2.89% loss), and the third step (at 157.21–261.30 °C with 17.64% loss) confirms the successful preparation of N-Al2O3@Pb(II)-2,4-DiNA.
3.3 SEM characterization
The synthesized materials were examined by the scanning electron microscope as shown in Fig. 4. Figure 4a represents the SEM image of N-Al2O3 which appears as homogenous particles with average range (18.74–26.50 nm), while Fig. 4b illustrates the image of Pb(II)-2,4-DiNA and this material was found in the nanometric range between 22.84 and 25.14 nm. Finally, the SEM image of N-Al2O3@Pb(II)-2,4-DiNAis provided in Fig. 4c with an average particle size in the range 18.74–26.23 nm.
3.4 EDX analysis of Pb(II)-2,4-DiNA
The electron-dispersive X-ray indicates the presence of the main elements C, N, O, and Pb in the newly synthesized Pb(II)-2,4-DiNAwith the following percentage values 33.64,26.86, 37.55 and 1.95%, respectively, as shown in Fig. 5 and listed in Table 3.
3.5 Electron spin resonance spectra ESR
ESR is an imperative nondestructive procedure used for recognizing the unpaired electrons formed due to the external effects on the matter caused by irradiation. Therefore, ESR methodology can give information about the stability prediction and the compressive strength of materials and their structures [26, 27]. The ESR signal intensity depends impressively on the number of free radicals or unpaired spins [26, 27]. Figure 6 shows the effect of progressive gamma radiation doses of 2, 10, and 16 kGy on the ESR spectra of N-Al2O3@Pb(II)-2,4-DiNA powder. Sharp ESR signals have been detected before and after the three irradiation doses giving approximately the same positions and intensity with a stable g-factor value around 2.
Figures 7 and 8 show the ESR spectra of the prepared thin films 1 and 2, respectively, before and after irradiation with 16 kGy. High and similar broad ESR signals were observed for the two thin films. The observed line broadening of signals refers to the dipole interactions between the paramagnetic ions joined to the magnetic dipole and exchange interactions [28]. The regular line shapes at ~ g = 2.32–2.35 obtained in the two thin films can be correlated to the octahedral and tetrahedral coordination states of the heavy atomic mass of Pb(II) ions [29], in addition to the strong tetrahedral coordination of AlO4 groups. After irradiating films with 16 kGy, no obvious changes either in the peak positions, intensity, or g-values were detected. This trivial effect of gamma irradiation on the prepared thin films refers to the high stability of such compacted structures against gamma irradiation because of the shielding effect of heavy Pb(II) and Al(III) ions and impede the processes of breaking bonds or forming free radicals because of irradiation.
Although N-Al2O3@Pb(II)-2,4-DiNA powder reveals stable behavior against the progressive gamma radiation as shown in Fig. 6, the prepared thin films can achieve this target more obviously since the solidification and hardening of materials give more compacted and blocked structures. Therefore, inhibition of photons passage takes place until they miss their energies through molecules without breaking out bonds or forming more free radicals [29,30,31]. This behavior indicates the acceptable shielding effect of the two prepared thin films to relatively low doses of ionizing radiation and their promising ability as radiation shielding materials [29,30,31].
3.6 Shielding study
The LAC values of the samples were obtained from the Phy-X/PSD software and from experimental measurements using gamma spectroscopy according to incident photon intensity (I0), attenuation (I) and glass thickness x (cm) at an energy of 662 keV(from radioactive point sources Cs-137and Co-60 (activity = 5 μCi) as shown in Fig. 9. It is clear from Fig. 9 that LAC of the samples depends on the concentration of Pb in the sample and the incident gamma ray energy. Also, Fig. 9 reveals that the experimental and theoretical values (by Phy-X/PSD software) of LAC are closed together which supports the data dependence of Phy-X/PSD software. The deviations between the results varied between 0 and 8.1% and the results are in good agreement.
For photon energies ranging from 0.05 to 1 MeV, the Phy-X software is used to calculate the gamma and X-ray radiation characteristics, such as the MAC values of the two samples, as shown in Fig. 10. It is observed from Fig. 10 that MAC values for S1 and S2 vary from 0.821 and 1.993 (cm2/g) at 0.05 MeV and from 0.064 to 0.065 (cm2/g) at 1 MeV, where MAC decreases with the increase in gamma-ray energy. Because photoelectric absorption is the dominant constituent at low energy, absorption is large. The attenuation coefficient is lowered when absorption is reduced. The photoelectric absorption edge values of S1 and S2 at photon energy 100 KeV, where photoelectric absorption dominates and MAC values (0.579 and 1.320 cm2/g, respectively) are very high and drop after these edges [30]. The MAC value at 0.662 MeV is 0.08 (cm2/g) for S1 and 0.083 (cm2/g) for S2, whereas the two samples have a much higher MAC (cm2/g) compared to the corresponding hematite-serpentine (0.079) [31], granite (0.074) [32] and PVC-H30 (0.0795) [33].
REP % is another important parameter, that offers essential information about the shielding performance of materials as shown in Fig. 11 at the same MAC energies. S1 and S2 RPE % values vary from 89 and 99% at 0.05 MeV and from 16.12 to 20% at 1 MeV, respectively; while at 0.662 MeV, they vary from 20 to 25%, which is better than SBR3 (19.5%) [21]. As a result, it is appropriate for use as a shield against X-rays and low-gamma energy.
The Zeff is another important aspect that helps in determining the attenuation capabilities of samples. The Zeff values for total interaction in S1 and S2 were plotted against the applied energy levels in Fig. 12. This graph shows that the effective number grows at first, but then decreases, resulting in a peak-like shape curve. The effective atomic numbers of the tested samples were larger at the low energy zone (owing to the photoelectric effect), progressively decreased, and became constant in the high energy region (due to pair production). The value of Zeff for S2 at photon energy 0.662 MeV is 13.1 which is greater than that of bismuth silicate glasses with BaO [27].
Figures 13 and 14 show the effective electron density (Neff) and effective conductivity (Ceff) of the investigated samples at various energies. These figures show the same trend as in Fig. 12, where both Neff and Ceff decreased as the energy increased. The obtained results show a remarkable peak for the two samples at 0.100 MeV demonstrating the K-shell absorption edges. Photoelectric absorption is the primary effect at low energies, but as energy increases, more photons are expelled, increasing the number of excited electrons and causing the observable reduction [34,35,36,37]. Since the effective conductivity (Ceff) is primarily determined by the number of collisions of energetic photons with electrons and the conversion of these collisions to free electrons, it is directly connected to Neff. According to the most recent results, S2 has the highest Neff and Ceff values. This is backed by the concept that Pb occupying S2 is greater than that in S1 as listed in Table 4. Pb acts as a modifier producing a stronger polymer structure and preventing their electrons from being easily excited. On the other hand, Pb can be incorporated into polymer structures as an intermediate ion giving a more compressed structure that avoids the passage effect of ionizing radiation [38].
The fast neutron-shielding properties of the investigated samples were manually estimated and reported in Table 5. They depict the macroscopic cross-sections for absorption (Ʃa), coherent scattering (Ʃco) and incoherent scattering (Ʃinco) of thermal neutrons (at energy = 0.025 eV) for the two investigated samples. The Ʃco for samples is larger than the Ʃinco and Ʃa, indicating that the examined samples under consideration have a high ability to scatter thermal neutrons. It is also noted that the Ʃco values for S1 are bigger than those for S2. Therefore, S1 has higher neutron absorption efficiency than S2. It is preferable to mix light and heavy elements in shield composites for optimum fast neutron attenuation. The listed data in Table 5 also show the fast neutron removal cross-sectional ƩR values, where the sample S1 has a much higher ƩR than S2 to indicate a minimal improvement in the neutron-shielding performance for S1 than S2, which is a better behavior compared to some standard shielding materials such as water = 0.102 cm−1, graphite = 0.077 cm−1, ordinary concrete = 0.094 cm−1and hematite-serpentine concrete = 0.097 cm−1 [31,32,33].
It has recently been discovered that charged particles like photons, show radiobiological effects, and cause defects in shield structures. They must have a sufficient attenuator for the safety of radiation workers. Therefore, determining the MSP and their ranges in the shield is very important [39]. Figure 15 demonstrates the MSP and range of proton (H-ions) and alpha (He-ions) with kinetic energy intervals 0.01–10 MeV for S1 and S2 using the SRIM code. MSP refers to the reduction of the kinetic energy (KE) of the ionization particles, which also refers to a decrease in passing through the examined samples. According to Fig. 15a, MSP for proton values increases quickly with increasing proton kinetic energy (KE) from 0.010 MeV reaching maximum values of 0.661 and 0.458 (MeV cm2/mg) at 0.08 MeV for S1 and S2, respectively. MSP values declined significantly as KE increased. Furthermore, the values MSP of S2 values is lower than S1. As seen in these figures, proton MSP levels increase and then decrease as kinetic energy increases. As observed in Fig. 15b, the MSP for the alpha value increased rapidly with increasing alpha kinetic energy (KE) from 0.01 MeV reaching maximum values of 1.741 and 1.287 (MeV cm2/mg) at 0.6 MeV for S1 and S2, respectively, thus, the values of MSP of alpha for the S2 value is less than the S1 value.
Figure 16a depicts the estimated proton range values for S1 and S2, with proton kinetic energy ranges from 0.01 to 10 MeV. The results demonstrate that S2 has relatively greater range (0.110–28.47 µm) values than S1 (0.112–28.3 µm) at low KE (0.01–1.8 MeV), but it has smaller range values (33.61–497.95 µm) than S1 (33.62–531.7 µm) at high KE (2–10 MeV). On the other hand, Fig. 15b represents the alpha range with a kinetic energy range 0.01–10 MeV, the values of the project range are from 0.073 to 0.074 (µm) at 0.01 MeV for S1 and S2, respectively, and from 49.11 to 48.82 (µm) at 10 MeV. The projected range represents the values for proton slowness in depth whereas, the current samples estimated range is greater than that of ordinary concrete and hematite serpentine concrete [34]. Because alpha particles have a higher mass (6.645 9 10–27 kg) than protons (1.673 9 10–27 kg), they have a lower velocity, allowing alpha particles to reach maxima at higher KE than protons. Furthermore, MSP values for alpha and protons decreased for all selected KEs, with S2 (with the highest Pb concentration) having the lowest values rather than the other sample S1.
The ion beam irradiation of polymeric materials results in the formation of highly reactive intermediates through a variety of reactions that result in rearrangements and/or formation of new bonds. One type of radiation damage to materials can be observed after irradiating the material with energetic ions. Damage caused by the constant displacement of current lattice atoms from their original positions is caused by energy transfer during nuclear collisions [38].
For the ion energies considered here, the damage is mainly caused by ions transferring energy to the target nucleus, which causes atomic displacement [39]. The damage energy profile can be calculated as the difference between the energy transferred to recoil and the energy lost to recoil at each increment depth in the E2RECOIL.TXT and IONIZ.TXT files for fast TRIM [40].
Quick TRIM modes projected damaging energy for 1 MeV H-ions and He-ions in examined samples, as shown in Fig. 17a, b. The difference in damage values for S1 and S2 at the same depths shows a depth shift. S1 damaging energy profiles are shifted to lower depths than S2. The damaging energy curve for H-ions has a peak at 11.6 µm with damage energy 0.57 (KeV/µm-Ion) for S1, and 11.8 µm with damage energy 0.47 (KeV/µm-Ion) for S2. A similar damage trend is projected for He-ions, with the damage peak at depth 2.6 µm with damage energies 0.857 and 0.840 (KeV/µm-Ion) for S1 and S2, respectively.
4 Conclusion
In this study, two thin films were assembled by the encapsulation of N-Al2O3@Pb(II)-2,4-DiNA nanocomposite into hydrolyzed polyurethane and hydrolyzed polyvinyl chloride. The ESR spectra of the two thin films exhibited relative stability against irradiation up to 16 kGy of γ-radiation. The two films were also examined by theoretical computational software for γ-ray shielding. The results indicated that S2 with the higher concentration of Pb ions showed the greatest LAC, MAC, Zeff, Neff and Ceff values. Also, the values of Ʃco for S1 showed higher neutron absorption efficiency than S2, however, S2 was found to have a higher ƩR than S1. This indicated a minimal improvement in the neutron-shielding performance of S2.The SRIM code was used to calculate MSP and the range of proton (H-ions) and alpha (He-ions) kinetic energy intervals (0.01–10 MeV) for S1 and S2 where the MSP for S2 was found to be less than that of S1. Furthermore, the values of the proton and alpha-particle ranges in S1 and S2 were slightly different. TRIM, a subroutine of the SRIM code was also used to investigate the surface changes in S1 and S2 based on irradiation with hydrogen and helium ions. The damage profile was examined as a function of dpa, and it was confirmed that for H-ions and He-ions, S2 exhibited greater displacement damage than S1. The net results from this work can approve the possible implementation of these thin films in radiation shielding applications.
Data availability
Relevant research data are included in the text of the work.
References
F.M. Clegg et al., Building science and radiofrequency radiation: what makes smart and healthy buildings. Build. Environ. 176, 106324 (2020)
H. Heidbuchel et al., Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace 16(7), 946–964 (2014)
W.D. Newhauser, R. Zhang, The physics of proton therapy. Phys. Med. Biol. 60(8), R155 (2015)
K. Dworecki et al., Modification of electrical properties of polymer membranes by ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B 166, 646–649 (2000)
A. Badriah, A.H. Almuqrin, M.S. Koubisy, E.A. Abdel Wahab, K.A. Mahmoud, K.S. Shaaban, M.I. Sayyed, Effect of Fe2O3 doping on structural, FTIR and radiation shielding characteristics of aluminium-lead-borate glasses. Prog. Nucl. EnergyNucl. Energy 141, 103931 (2021)
R.A. Abu Saleem et al., Radiation shielding of fiber reinforced polymer composites incorporating lead nanoparticles—an empirical approach. Polymers 13(21), 3699 (2021)
M.A. Alothman et al., Significant influence of MoO3 content on synthesis, mechanical, and radiation shielding properties of B2O3-Pb3O4-Al2O3 glasses. J. Alloys Compds. 882, 160625 (2021)
Z.A.I.M. Nimet, O. Bayhatun, A study on the gamma-ray attenuation coefficients of Al2O3 and Al2O3 TiO2 compounds. Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi 22, 312–318 (2018)
M.J.R. Aldhuhaibat et al., Radiation attenuation capacity improvement of various oxides via high density polyethylene composite reinforcement. Ceram. Int. 48(17), 25011–25019 (2022)
P. Sigmund, A. Schinner, Note on measuring electronic stopping of slow ions. Nucl. Instrum. Methods Phys. Res. Sect. B 410, 78–87 (2017)
H.H. Andersen, P. Sigmund, Stopping of heavy ions: a topical issue. Nucl. Instrum. Methods Phys. Res. Sect. B 195(1–2), 1–2 (2002)
J.F. Ziegler, M.D. Ziegler, J.P. Biersack, SRIM–the stopping and range of ions in matter. Nucl. Instrum. Methods Phys. Res. Sect. B 268(11–12), 1818–1823 (2010)
J.F. Ziegler, M.D. Ziegler, J.P. Biersack, SRIM—the stopping and range of ions in matter (SRIM Co., Chester, 2008)
A. Bozkurt, Monte Carlo calculation of proton stopping power and ranges in water for therapeutic energies. EPJ Web Conf. 154, 01007 (2017)
A.M. Madbouly, A.A. El-Sawy, Calculation of gamma and neutron parameters for some concrete materials as radiation shields for nuclear facilities. Int. J. Emerg. Trends Eng. Dev. 3(8), 7–17 (2018)
E.M. Abou Hussein, A.M. Madbouly, F.M. Ezz Eldin, N.A. ElAlaily, Evaluation of physical and radiation shielding properties of Bi2O3–B2O3 glass doped transition metals ions. Mater. Chem. Phys. 261, 124212 (2021)
E.M. Abou-Hussein, A.M. Madbouly, F.M. Ezz-Eldin, Characterization of some radiation shielding, optical, and physical properties of fluorophosphate glasses modified by Sm3+. J. Mater. Sci. Mater. Electron. 32(21), 25933–25951 (2021)
H. Paul, The stopping power of matter for positive ions, in G. Natanasabapathi (ed.) Modern Practices in Radiation Therapy (InTech, 2012), p. 113–133. http://www.intechopen.com/books/modern-practices-in-radiation-therapy/the-stopping-power-of-matter-forpositive-ions (ISBN: 978-953-51-0427-8, chapter 7)
E. Sakar, Ö.F. Özpolat, B. Alım, M.I. Sayyed, M. Kurudirek, Phy-X /PSD: development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem.. Phys. Chem. 166, 108496 (2020)
Ç. Nuhoğlu, et al. Resistivity, ESR, and radiation shielding properties of the volcanic rock materials. Adv. Condens. Matter Phys. 2014 (2014)
M.T. Abou-Laila et al., Gamma irradiation effects on styrene butadiene rubber/Pb3O4: mechanical, thermal, electrical investigations and shielding parameter measurements. Radiat. Phys. Chem. Phys. Chem. 192, 109897 (2022)
L. Zhang et al., Decontamination of U (VI) on graphene oxide/Al2O3 composites investigated by XRD, FT-IR and XPS techniques. Environ. Pollut.Pollut. 248, 332–338 (2019)
K. Srinivasan, T. Stalin, Studies on inclusion complexes of 2, 4-dinitrophenol, 2, 4-dinitroaniline, 2, 6-dinitroaniline and 2, 4-dinitrobenzoic acid incorporated with β-cyclodextrin used for a novel UV absorber for ballpoint pen ink. J. Incl. Phenom. Macrocycl. Chem. 78(1), 337–350 (2014)
B. Gierczyk et al., 15N NMR and FTIR studies of 2, 4-dinitroanilines and their salts. J. Mol. Struct. 524(1–3), 217–225 (2000)
T. Stalin et al., Preparation and characterizations of solid/aqueous phases inclusion complex of 2, 4-dinitroaniline with β-cyclodextrin. Carbohydr. Polym.. Polym. 107, 72–84 (2014)
E.M. Abou-Hussein, A. Abdel-Galil, Synthesis, optical, chemical and electrical characterizations of γ-irradiated transition metal ions reinforced borate glasses. J. Non-Cryst. SolidsCryst. Solids 610, 122302 (2023)
E.M. Abou Hussein, A.M. Madbouly, N.A. El Alaily, Gamma ray interaction of optical, chemical, physical behavior of bismuth silicate glasses and their radiation shielding proficiency using Phy-X/PSD program. J. Non-Cryst. SolidsCryst. Solids 570, 121021 (2021)
D. Moncke, D. Ehrt, in Materials Science Research Horizons, ed. by H.P. Glick (Nova Science Publishers, Inc., New York, 2009), p. 1
J.E. Shelby, Introduction to glass science and technology, 2nd edn. (Royal Society of Chemistry, Cambridge, 2005)
M. Kurudirek, Heavy metal borate glasses: potential use for radiation shielding. J. Alloys Compd. 72, 1227–1236 (2017)
I.I. Bashter, Calculation of radiation attenuation coefficients for shielding concretes. Ann. Nucl. Energy 24, 1389–1401 (1997)
H.O. Tekin et al. An investigation on shielding properties of different granite samples using MCNPX code. Digest J. Nanomater. Biostruct. (DJNB) 13(2) (2018)
K.A. Mahmoud et al., Investigation of the gamma ray shielding properties for polyvinyl chloride reinforced with chalcocite and hematite minerals. Heliyon 6(3), e03560 (2020)
R. Bagheri, A.K. Moghaddam, H. Yousefnia, Gamma ray shielding study of barium–bismuth–borosilicate glasses as transparent shielding materials using MCNP-4C code, XCOM program, and available experimental data. Nucl. Eng. Technol.. Eng. Technol. 49(1), 216–223 (2017)
R.M. El-Sharkawy et al., Investigation of mechanical and radiation shielding characteristics of novel glass systems with the composition xNiO-20ZnO-60B2O3-(20–x) CdO based on nanometal oxides. J. Non-Cryst. SolidsCryst. Solids 528, 119754 (2020)
E.A. Allam et al., Enhancement and optimization of gamma radiation shielding by doped nano HgO into nanoscale bentonite. Nucl. Eng. Technol.. Eng. Technol. 54(6), 2253–2261 (2022)
R.M. El-Sharkawy et al., Synergistic effects on gamma-ray shielding by novel light-weight nanocomposite materials of bentonite containing nano Bi2O3 additive. Ceram. Int. 48(5), 7291–7303 (2022)
W. Wesch, E. Wendler, Ion beam modification of solids, in Ion-Solid Interaction and Radiation Damage, Springer Series in Surface Sciences, vol. 61 (2016).https://doi.org/10.1007/978-3-319-33561-2(ISBN 978-3-319-33561-2)
W.J. Weber, Y. Zhang, Predicting damage production in monoatomic and multi-elemental targets using stopping and range of ions in matter code: challenges and recommendations. Curr. Opin. Solid State Mater. Sci.. Opin. Solid State Mater. Sci. 23, 100757 (2019)
R.E. Stoller, M.B. Toloczko, G.S. Was, A.G. Certain, S. Dwaraknath, F.A. Garner, On the use of SRIM for computing radiation damage exposure. Nucl. Inst. Methods Phys. Res. B 310, 75–80 (2013)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
EMAH, AMM, EAA, MEM conceptualization, methodology, software, validation, investigation, data curation, writing—review and editing, visualization, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, E.M.A., Madbouly, A.M., Allam, E.A. et al. Thin-film assembly via encapsulation of nano-aluminum oxide-linked-pb(II)-2,4-dinitroaniline complex into a polymeric matrix as efficient radiation shielding materials. Appl. Phys. A 129, 747 (2023). https://doi.org/10.1007/s00339-023-07013-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07013-1