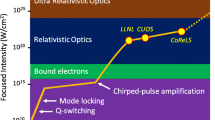
Abstract
Slightly more than 60 years have passed since the introduction of the laser. The unique property of high peak power in short pulses has led to applications in which light energy replaces mechanical energy for removing mass, structuring surfaces, creating new materials, weapons, remote analysis, fusion, surgery, and many other esoteric applications that fall under the process called laser ablation. This manuscript addresses several accomplishments in laser ablation research and development, including fundamental behavior, some unique applications with emphasis on chemical analysis, and a current interest to measure isotope ratios in laser induced plasmas at atmospheric pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction: 60+ years
Considering that the laser was called a ‘solution in search of an application,’ we have seen the benefits of creative minds when innovative technology is presented by (and to) the scientific community. Numerous applications for the laser immediately were proposed, applications based on the unique properties of optical purity, directionality, and high peak power in short energy pulses—ablation. Laser ablation research began shortly after the invention of the laser in 1960 [1], 60+ years of R&D. It would be impossible to summarize the numerous applications or to thoroughly delve into the fundamental mechanisms that have been attributed to ablation. There are many books, journal articles and dedicated conferences addressing the stellar accomplishments laser ablation research and development [2,3,4,5,6,7,8,9,10]. Only a few references are presented here as the reader can easily perform a literature search to finds thousands of related studies. Laser ablation is simple and complicated; the paradox is the difference in utilizing ablation for an application versus understanding ablation for an application, the former requiring reproducibility in most cases and the latter requiring new knowledge. This manuscript emphasizes chemical analysis applications that I have pursued with some connection to fundamental studies. For me, it has been an amazing journey—as a student first seeing a laser beam (similar to a laser light show but without the safety of today) and then serendipitously spending almost 50 years enamored by tiny explosions created from focused pulsed laser beams.
Ablation is the word that the scientific community has chosen to describe an unknown process when a pulsed (high power CW lasers can remove mass under more controlled or understood thermodynamics) laser beam explodes a portion of a material (generally a solid, although liquids and gases can be ablated) to transform a portion of that material into a transient high temperature vapor plume (plasma). I have often used the term ‘laser material interaction’ to avoid the implication of a mechanism. From first light on a material, the flood of photon energy in time is overwhelming for the material to know what hit it. Based on atom/electron excitation by the photons to cessation of the event (calmness after the storm), the time period can cover many orders of magnitude. The international scientific community has intensely investigated fundamental mechanisms to understand and predict this intriguing transient physic and chemistry phenomenon. Throughout the 60+ ears of research, most known physical and chemical processes have been tested for relevance, without to best of my knowledge reveling the beauty of this technology but instead exposing it complexity [11,12,13,14,15,16,17,18,19,20]. Figure 1 lists some of the mechanisms that have been hypothesized to underlie laser ablation, which mechanism or combination of mechanisms is operative is significantly dependent on the laser irradiance (power density used interchangeably), the energy/area-time. Figure 2 shows examples of physical measurements from experiments in which the variable was the laser pulse duration—pulses with 6 ns versus 100 femtoseconds (change in irradiance) and all other parameters (wavelength, energy, spot size, number of pulses, sample material) kept constant [21,22,23,24]. As can be seen from crater profiles, aerosol size distribution, plasma persistence, and analytical measurements of mass ablation rate, there are significant changes in ablation behavior based on irradiance. Although the wavelength, spot size and energy were the same, ablation is not the same as the irradiance is several orders greater for the femtosecond compared to nanosecond pulsed experiments. The amount of mass ablated changed as did the particle size and particle character. Even the particle chemistry will change with pulse duration as the plasma cooling rate changes. It is clear that everything is not the same just because the wavelength, energy density and sample are the same. For example, optical penetration depth changes due to multiphoton absorption even though the wavelength is the same.
Examples of experimental measurements in which irradiance was changed by using a 100 fs versus a 6 ns pulsed laser beam while keeping all other parameters constant. A Crater profiles in brass using a white-light interferometer with depth profiling, B the difference in particle size distribution in the ablated aerosol from ablation of NIST 1711 aluminum alloy. C the persistence of the laser plasma measured using the copper spectral line intensity. D ICP-MS measurements of zinc intensity (log scale). The width of each line indirectly represents the particle size distribution
In Fig. 3, LA is presented as a series of non-linear/linear events with examples of how plasma temperature, electron number density and crater depth can suddenly change, and then follow linearity based on irradiance [21,22,23,24]. Thresholds for other esoteric events occur at much higher irradiances, for example phase explosion, laser fusion and others. A material can undergo classical thermodynamic processes (melting, boiling, and vaporization) but in this case, one could calculate the outcome—how much material was removed and transitioned to the gas phase. However, this would not be classified as ablation in which ‘non-thermodynamic’ behavior, is expected and the outcome cannot be fundamentally predicted. Ablation could be considered similar to a nuclear explosion—an enormous amount of energy on a target in a short period of time; several researchers are investigating LA behavior to model explosions [25, 26]. Maybe it will be possible that nuclear explosion models can explain laser ablation behavior [27].
A Drawing representing laser ablation process showing white-light plasma, heating of the solid surface, and particles in the aerosol. B A representation of non-linearly/linear behavior expected based on irradiance. C The measured plasma temperature and electron number density using a nanosecond pulsed laser for ablation of a copper sample, with an abrupt change at approximately 20 GW/cm2. D The change in measured crater depth (log scale) and profile for a single pulse at the same irradiances as show in C
From the list in Fig. 1 (which is not all-inclusive), it is not known which collection of mechanisms are dominant for an application with many coupled parameters (material and laser) governing the process. Understanding the complexity of ablation is intertwined with the idiosyncrasies of the detection modality, which can easily compound the interpretation. These statements are not meant to be discouraging, but a challenge to the scientific community to unravel this dynamic non-linear process. Significantly, these comments about complexity in fundamentals do not take away from the tremendous growth of laser ablation applications. Applications can be to process the ablated material itself, like surface texturing, cutting, medical (surgery), discover meta-stable material phases that are formed in the high temperature and rapidly cooling plasma plume (nano-particle formation), pulsed laser deposition (PLD), plasma emission spectroscopy, and many others [28,29,30,31,32,33,34,35,36,37,38]. Fundamental studies are imperative for predicting the process, and they still are addressed as there are no first-principle models to describe the ablation process or predict performance for many ablation applications. By using fixed conditions, one can replicate the outcome and, in some cases, model the process with fitting parameters. A current literature search on laser ablation shows the transition from fundamental research to empirical development. Funding for fundamental research exists in very specialized areas but most research is dedicated to application demonstration and optimization. Although laborious, improvements in many applications can be gleaned faster through empirical efforts. Empirical studies have the goal to achieve enhanced performance and reproducible behavior—the backbone for most industrial applications. For example, there are approximately 700,000 LASIK surgeries per year although no first principle model exists to predict the amount of ablation. The process is reproducible of course. One of the key questions in laser ablation research is—how much mass will be ablated. The question has no simple answer as the amount depends critically on the laser and material parameters.
2 Spectroscopy
Spectroscopy (optical and mass) has served a dual purpose in ablation research and development. Optical emission measurements provide fundamental knowledge of the laser-induced plasma at (in) the sample (temperature, electron number density, etc.) and the luminous optical plasma spectroscopically represents the elemental and isotopic content of the material being ablated. The first published account of ablation for optical spectroscopy is an abstract of a talk given by Breech in 1962 [39]. Mass spectrometers with ablation serve both to understand ionization behavior and mass to charge ratio for analysis. Laser ablation as an ion source for mass spectrometry first appeared by Honig and Woolston [11]. Miller and others summarized early laser ablation R&D including studying the fundamental properties and analytical applications based on spectroscopy in several laser ablation manuscripts [7, 9, 19, 21, 24]. Many fundamental processes in the ablation plasma have been established by optical time-resolved spectroscopy measurements.
3 Chemical analysis
Laser ablation is an ideal approach for the analytical community to use light energy to replace mechanical and chemical energy. The traditional approach to analysis is to digest samples (solids) in acids, and although archaic and cumbersome, digestion is still widely used today. Even though the 1962 paper presented the new opportunity [39], technology adoption takes decades. The application for chemical analysis herein deals with ablating a sample at atmospheric pressure (not inside a mass spectrometer). Two common configurations for LA chemical analysis include optical spectroscopy and mass spectrometry [40,41,42,43,44,45,46,47,48,49,50]. Figure 4 shows an example configuration for a Tandem instrument approach to chemical analysis in which the plasma photons are measured simultaneously with the ablated aerosol that is transported to an ICP (inductively coupled plasma using MS (mass emission spectrometry) or OES (optical emission spectroscopy) for detection. The optical-based chemical analysis application (measuring the light at the sample surface) is commonly referred to as laser induced breakdown spectroscopy (LIBS). The atomic and ionic emission wavelengths represent the samples’ elemental content and the intensity of the emission lines represents their concentrations. The choice of detection modality (measuring photons in the plasma or transporting aerosol to an ICP) depends on the requirements of the analysis—which elements and isotopes in a sample need to be measured and at what concentration levels. The analysis requirements also govern the optimum laser parameters (energy, pulse duration, irradiance). In 1982 arriving at the Lawrence Berkeley National Laboratory a high-power laser was available and there was a potential application that could be marketed to the DOE (Department of Energy)—cleanup of legacy waste sites that had tons of contaminated solid materials requiring analysis before disposal. Thanks to the DOE for recognizing the potential of LA, a 25+ year fundamental, focused-applied research project was supported to understand and develop LA using LIBS and ICP for this application. Through years of R&D, the international analytical community has established metrics for accurate and precise elemental and isotopic analysis for almost every element on the periodic chart.
Diagram showing a Tandem laser ablation sampling instrument configuration with simultaneous measurements of optical emission spectroscopy using LIBS and LAMIS, with aerosol transport to an ICP. Any combination of detectors can be used depending on the elements and isotopes of interest to be measured
LIBS was not adopted for many years because of the instability of the ablation process, due in large part to the instability of early lasers. Criticism included that the process was too irreproducible and no standards existed, it was challenging to perform quantitative analysis and the approach was subject to matrix effects (ablation behavior dependent on the chemical and physical properties of the sample). Although a transient laser plasma is not an ideal atomic emission source, arc and spark sources are widely utilized in industry. The laser allows spatial analysis (mapping), and any sample can be ablated compared to an arc/spark-based instrument. To bypass the early challenges of LIBS, the approach was not to use the plasma emission but instead send the ablated aerosol into a secondary stable analytical source and everything was tried, flames, arcs, microwaves without real interest until the ICP. The ablated aerosol contains the mass in the form of ejected particles (spallation), atoms, ions, and as the plasma cools, the nucleation and condensation of nanoparticles. Particles from spallation versus nucleation and condensation will have a diverse size range, the size distribution established by the laser wavelength, pulse duration, energy density on the material and the material properties. Optimization of the laser parameters for material classes can produce particles with a narrow nanometer size distribution. Of course, laser ablation is recognized as a technology for fabrication of nanomaterials. For years, a general belief was that the ICP was the panacea for LA based chemical analysis, blast away and the ICP would efficiently digest and ionize the ablated aerosol. It was quickly realized that the ICP was responsible for many of the perceived problems with ablation; elemental fractionation was not from LA itself but a manifestation of the particle size distribution in the ICP. Many questioned how fractionation could be severe using LA for chemical analysis when stoichiometric materials could be fabricated using PLD. Numerous studies have been undertaken to improve the performance of LA-ICP-MS by investigating every adjustable parameter including laser pulse duration, wavelength, repetition rate, sampling mode (drilling versus raster scanning) and energy density. LA-ICP-MS has become a mainstay technology for geochemical age dating, direct solid sample qualitative and quantitative analysis, 2D/3D mapping, depth profiling, forensics, nuclear safeguards, and many industrial applications (examples in Fig. 5). Laser ablation for chemical analysis is a compelling value proposition—any sample, no sample preparation, minimal sample quantity, green, bulk or single particle analysis, spatial analysis (2D/3D mapping), multi-elemental/isotopic characterization, all this information from every laser ablation event on a sample.
Example analytical chemistry measurements using LIBS and LA-ICP-MS. A LIBS spectral intensity for Hg and Pb measured in a sample with the inset showing linear calibration. B An example of the H line intensity in a LIBS plasma as a measurement of water content. C The use of successive femtosecond laser pulsed to depth profile critical elements in a Li-ion battery electrode, with 7 nm depth resolution for this case. D An example of repetitively pulsed femtosecond laser ablation analysis of uranium particles on filter paper with analysis using ICP-MS sowing 235U and 238U isotopes, and excellent linearity in calibration with femtogram limits of detection. 5E is an example 2D/3D spatial mapping of chemistry. Spatial resolution is defined by the spot size of the laser beam and depth resolution is defined by the energy density of the laser and material properties
With modern-day computers and chemometrics algorithms, a broadband optical spectrum represents all elements in a sample (the matrix) essentially providing a barcode chemical identification for every material (Fig. 6). Similar to a product barcode, every item (natural, manmade) could be linked to a chemical barcode. One of the most value-added applications of LIBS is remote or standoff chemical analysis. Figure 7 shows a few examples of LIBS used for remote analysis [51,52,53]. LIBS gained significant attention when NASA utilized this technology on the Mars Curiosity Rover in 2011 [51]. To best of my knowledge, no other technology can provide elemental or isotopic analysis at distance. For industrial real-time monitoring of chemistry (in-line metrology), no other analytical technology provides the breath of elements that can be measured. The benefits of this technology are significant for many industrial measurements including energy (battery, solar), advanced manufacturing, environmental analysis, medical, materials, food safety, nuclear nonproliferation and essentially every aspect of society where chemistry is important in defining a material.
Examples of remote (standoff) LIBS. A and B Photos from an Applied Spectra, Inc. project to demonstrate LIBS for standoff detection of explosives at 50 m. C Taken from the NASA website (51). D The experimental concept showing classical diffraction limited focusing versus filamentation focusing using a high-power femtosecond pulsed laser. E Images of filaments produced at the Lawrence Berkeley National Laboratory (52). F An image of a femtosecond laser filament produced by the Teramobile project (53)
4 LIBS and LAMIS for isotopes
LIBS has primarily been addressed for elemental analysis, although several early papers demonstrated isotopic analysis particularly when working with the sample at reduced pressure [54]. Isotopic splitting is only a few picometers for most atomic and ionic spectral emission lines [55]. LIBS can be used for isotopic analysis at atmospheric pressure by using appropriate detector gating, delay time, and high-resolution spectrometers. Figure 8A shows an example time response for ionic, atomic, and molecular LIBS plasma emission, when ablating a boron nitride sample using a nanosecond pulsed laser. Ionic and atomic emission (Fig. 8 B) dominate initially after the laser pulse with the formation of molecular species (BO) later in time as the plasma cools. A technology developed by the LBNL and ASI scientists named LAMIS (laser ablation molecular isotopic spectroscopy) measures isotope ratios in laser plasmas at atmospheric pressure from molecular emission band spectra [56,57,58]. The concept is based on a manuscript from 1924 demonstrating isotope shifts of boron-oxide molecular band emission [59]. Figure 8B shows 10/11B atomic line 209 nm wavelength with a 2.5 pm isotopic split. The measurement was at low pressure; the lines would be difficult to resolve in an atmospheric pressure LIBS plasma. Figure 8C shows a LIBS spectrum for BO emission in an atmospheric pressure LIBS plasma. The 10/11BO split is 730 pm for this spectral emission, which is easily resolved. In addition, with molecular emission, a synthetic spectrum can be calculated and fit to the measured spectrum to extract the isotope ratio (isotope standards are not required for analysis). The energy level diagrams in Fig. 8B and C give an example of the underlying principles for atomic versus molecular isotope splitting.
Persistence of a laser induced plasma showing time behavior for ionic, atomic, and molecular emission after ablation of a boron nitride sample using a nanosecond pulsed laser (A). Boron oxide (BO) species form during recombination with oxygen in the ambient; two molecular bands are shown. B A diagram of electronic energy level splitting due to isotopic structure of the atom/ion with an example of an optical spectrum for B. C A diagram for molecular energy level transitions with rotational and vibrational structure related to isotope splitting, and a LIBS spectrum from BO emission
Figure 9 shows examples of LAMIS and LIBS spectra for extracting isotope information. Figure 9A and B are theoretical and measured LAMIS emission for SrO, respectively. Figure 9C shows 12/13C isotopes in C2 molecular spectra—both the synthetic and measured spectra. The structure on the lines is from the rotational emission as well as the background noise. LAMIS mitigates the small atomic and ionic line splitting problem, but in general, molecular emission in the plasma is not as intense as atomic and ionic emission lines, although efforts are underway to enhance molecular emission intensities in LIBS plasmas. Figure 9D shows a LIBS (ionic line emission) measurement of 235/238U isotopic splitting at 424.4 nm which has a 25 pm split and can be resolved using a high-resolution spectrometer and appropriate detector gate delay. In this case, the background is from other atomic and ionic lines in the spectral vicinity as well as the S/N (signal to noise). Lorentzian line fitting is used to extract the isotopic ratio. For LIBS and LAMIS measurements, the critical parameter for achieving good isotope ratio measurements is the S/N ratio; the better the fit, the more accurate and precise are the results.
Examples of emission spectra for isotopic measurements. A Calculated spectra for the A–X (2–0) molecular emission spectra for SrO, and B The measured LIBS/LAMIS spectra measured for three of the Sr isotopes. C The measured LIBS C2 molecular emission spectrum with the calculated (synthetic) spectrum. D A measured and fitted uranium 235–238 isotopic line pair from a LIBS spectrum
5 Laser ablation R&D: the next 60 years
The future is bright for laser ablation. The number of applications continues to increase led by improvements in commercial lasers and technology recognition. Early instrumentation could not meet the needs of industrial requirements; not because of the technology but because lasers were just being developed, were unreliable and did not have the high power of today’s lasers. Lasers with higher energy, shorter pulses and high repetition rates continue to become more efficient, reliable, and affordable. Laser ablation has been advanced (always more to come) to the point that it is now a routine approach for many industrial processes. It will be intriguing to see what new esoteric applications will be invented, as many of the applications of today were not envisioned when the laser was first developed. Machine learning and artificial intelligence will contribute to advancements in laser ablation applications, and possibly help in understanding the process. It also will be interesting to see if a particular application will justify the need for fundamental research to establish the underlying mechanisms of laser ablation, where an empirical approach does not make sense.
In my area of expertise, laser ablation is a twenty-first century approach to chemical analysis, and companies are seeing increased demand for laboratory-based commercial instruments to rapidly measure every element (and isotope) in a sample with excellent sensitivity, accuracy, and precision. In addition, laser ablation allows field-based chemical analysis applications, including real-time metrology in advanced manufacturing (batteries, solar cells) lines, standoff analysis during food and pharmaceutical production, remote sensing, and many other industrial processes where chemistry is critical to performance. Isotopic analysis using LIBS and LAMIS will continue to advance in applications that cannot be addressed by mass spectrometry [51,52,53, 60, 61]. Laser ablation changes the paradigm for chemical analysis—bring the lab to the sample instead of vice versa. NASA successfully demonstrated that LIBS instruments can be operated in remote harsh environments, much more challenging than most commercial industrial applications. The technology has moved beyond early adopters and becoming mainstay. Automation, machine learning and artificial intelligence will expedite adoption towards a non-user analytical experience.
Data availability
There are no other data required.
References
T.H. Maiman, Stimulated emission of radiation in ruby. Nature 187, 493–494 (1960)
J.F. Ready, Effects due to absorption of laser radiation. J. Appl. Phys. 36(2), 462–468 (1965)
M.J. Lubin, B. Yaakobi, Laser Interaction and Related Plasma Phenomena: Springer Series, vol. 7 (Springer, Berlin, 1971)
S. Charschan, Lasers in Industry (Van Nostrand Reinhold Company, New York, 1972)
Scott, Brian F. Laser machining and fabrication—a review, in Proceedings of the Seventeenth International Machine Tool Design and Research Conference (Palgrave, London, 1977), pp. 335–339.
Conference on Laser Ablation, 1991 – present.
J.C. Miller, A brief history of laser ablation, in AIP Conference Proceedings, 1993.
International High-Power Laser Ablation (HPLA) Conference, 1998–present.
C. Phipps, Laser Ablation and Its Applications, Springer Series in Optical Sciences, vol. 129 (Springer, Boston, 2007)
H. Hora, Laser Interaction and Related Plasma Phenomena (Springer Science, Berlin, 2012)
R.E. Honig, J.R. Woolston, Laser-induced emission of electrons, ions, and neutral atoms from solid surfaces. Appl. Phys. Lett. 2(7), 138–139 (1963)
J.F. Ready, Effects of High-Power Laser Radiation (Academic Press, Cambridge, 1971)
S. Küper, M. Stuke, Femtosecond UV excimer laser ablation. Appl. Phys. B 44(4), 199–204 (1987)
H. Hora, Plasmas at High Temperature and Density (Springer, Berlin Heidelberg, 1991)
M.D. Shirk, P.A. Molian, A review of ultrashort pulsed laser ablation of materials. J. Laser Appl. 10(1), 18–28 (1998)
S. Amoruso, R. Bruzzese, N. Spinelli, R. Velotta, Characterization of laser-ablation plasmas. J. Phys. B Atom. Mol. Opt. Phys. 32(14), 131 (1999)
T. Lippert, Laser application of polymers, in Polymers and Light. (Springer, Berlin, 2004), pp.51–246
I.N. Mihailescu, J. Hermann, Laser–plasma interactions, in Laser Processing of Materials. (Springer, Berlin, 2010), pp.49–88
J.C. Miller (ed.), Laser Ablation: Principles and Applications, vol. 28 (Springer Science & Business Media, Berlin, 2013)
P.K. Kaw, Nonlinear laser–plasma interactions. Rev. Mod. Plasma Phys. 1(1), 1–42 (2017)
R.E. Russo, X. Mao, O.V. Borisov, Laser ablation sampling. TrAC Trends Anal. Chem. 17(8–9), 461–469 (1998)
R.E. Russo, X. Mao, S.S. Mao, The physics of laser ablation in microchemical analysis. Anal. Chem. 74(3), 70A-77A (2002)
R.E. Russo, X.L. Mao, C. Liu, J. Gonzalez, Laser assisted plasma spectrochemistry: laser ablation. J. Anal. At. Spectrom. 19(9), 1084–1089 (2004)
R.E. Russo, X. Mao, J.J. Gonzalez, V. Zorba, J. Yoo, Laser ablation in analytical chemistry. Anal. Chem. 85, 6162–6177 (2013)
H. Hora, J.J. Schwarz, Laser interactions and related plasma phenomena. Nucl. Fusion 17(1), 165 (1977)
C. Kimblin, R. Trainham, G.A. Capelle, X. Mao, R.E. Russo, Characterization of laser-induced plasmas as a complement to high-explosive large-scale detonations. AIP Adv. 7(9), 095208 (2017)
E. Cockram. Laser ablation plasmas as surrogates of early nuclear fireball vapor chemistry. PhD diss., University of Florida (2022)
J.F. Ready, Industrial Applications of Lasers (Elsevier, Amsterdam, 1997)
M. Wolbarsht, Laser Applications in Medicine and Biology (Springer Science & Business Media, Berlin, 1991)
T. Lippert, A. Yabe, A. Wokaun, Laser ablation of doped polymer systems. Adv. Mater. 9(2), 105–119 (1997)
E. Schena, P. Saccomandi, Y. Fong, Laser ablation for cancer: past, present and future. J. Funct. Biomater. 8(2), 19 (2017)
A.F. Obilor, M. Pacella, A. Wilson, V.V. Silberschmidt, Micro-texturing of polymer surfaces using lasers: a review. Int. J. Adv. Manuf. Technol. 120, 103–135 (2022)
C.W. Schneider, T. Lippert, Laser processing of materials, in Laser Ablation and Thin Film Deposition, Springer Series in Materials Science, vol. 139, ed. by P. Schaff (Springer, Berlin, 2010), pp.89–112
K. Miyamoto (ed.), Plasma Physics for Controlled Fusion (Springer Series on Atomic, Optical, and Plasma Physics) (Springer, Berlin, 2016)
A. Giulietti (ed.), Laser-Driven Particle Acceleration Towards Radiobiology and Medicine (Biological and Medical Physics, Biomedical Engineering) (Springer, Berlin, 2016)
N.G. Semaltianos, Nanoparticles by laser ablation. Crit. Rev. Solid State Mater. Sci. 35(2), 105–124 (2010)
C. Phipps, W. Bohn, T. Lippert, A. Sasoh, W. Schall, J. Sinko. A review of laser ablation propulsion, in AIP Conference Proceedings, vol. 1278, no. 1, pp. 710–722. American Institute of Physics (2010)
D.S. Zhang, Z.G. Li, C.H. Liang, Diverse nanomaterials synthesized by laser ablation of pure metals in liquids. Sci. China Phys. Mech. Astron. 65(7), 1–35 (2022)
F.J.A.S. Brech, Optical microemission stimulated by a ruby laser. Appl. Spectrosc. 16(2), 59 (1962)
E.F. Runge, R.W. Minck, F.R. Bryan, Spectrochemical analysis using a pulsed laser source. Spectrochim. Acta 20(4), 733–736 (1964)
W. Van Deijck, J. Balke, F.J.M.J. Maessen, An assessment of the laser microprobe analyzer as a tool for quantitative analysis in atomic emission spectrometry. Spectrochim. Acta Part B 34(9–10), 359–369 (1979)
L.J. Radziemski, From LASER to LIBS, the path of technology development. Spectrochim. Acta Part B Atom. Spectrosc. 57(7), 1109–1113 (2002)
M. Thompson, J.E. Goulter, F. Sieper, Laser ablation for the introduction of solid samples into an inductively coupled plasma for atomic-emission spectrometry. Analyst 106(1258), 32–39 (1981)
J.W. Carr, G. Horlick, Laser vaporization of solid metal samples into an inductively coupled plasma. Spectrochim. Acta Part B Atom. Spectrosc. 37(1), 1–15 (1982)
A.L. Gray, Solid sample introduction by laser ablation for inductively coupled plasma source mass spectrometry. Analyst 110(5), 551–556 (1985)
D. Günther, S.E. Jackson, H.P. Longerich, Laser ablation and arc/spark solid sample introduction into inductively coupled plasma mass spectrometers. Spectrochim. Acta Part B 54(3–4), 381–409 (1999)
L.J. Radziemski, Review of selected analytical applications of laser plasmas and laser ablation, 1987–1994. Microchem. J. 50(3), 218–234 (1994)
V. Margetic, A. Pakulev, A. Stockhaus, M. Bolshov, K. Niemax, R. Hergenröder, A comparison of nanosecond and femtosecond laser-induced plasma spectroscopy of brass samples. Spectrochim. Acta Part B 55(11), 1771–1785 (2000)
C. Liu, X.L. Mao, S.S. Mao, X. Zeng, R. Greif, R.E. Russo, Nanosecond and femtosecond laser ablation of brass: particulate and ICPMS measurements. Anal. Chem. 76(2), 379–383 (2004)
J. Koch, D. Günther, Review of the state-of-the-art of laser ablation inductively coupled plasma mass spectrometry. Appl. Spectrosc. 65(5), 155A-162A (2011)
NASA website. https://mars.nasa.gov/msl/home/
H. Hou, G.C.-Y. Chan, X. Mao, R. Zheng, V. Zorba, R.E. Russo, Femtosecond filament-laser ablation molecular isotopic spectrometry. Spectrochim. Acta Part B Atom. Spectrosc. 113, 113–118 (2015)
J. Kasparian, M. Rodríguez, G. Méjean, J. Yu, E. Salmon, H. Wille, R. Bourayou et al., White-light filaments for atmospheric analysis. Science 301(5629), 61–64 (2003)
W. Pietsch, A. Petit, A. Briand, Isotope ratio determination of uranium by optical emission spectroscopy on a laser-produced plasma-basic investigations and analytical results. Spectrochim. Acta Part B 53(5), 751–761 (1998)
Go. BreÍt, Theory of isotope shift. Rev. Mod. Phys. 30(2), 507 (1958)
R.E. Russo, A.A. Bol’shakov, X. Mao, C.P. McKay, D.L. Perry, O. Sorkhabi, Laser ablation molecular isotopic spectrometry. Spectrochim. Acta Part B 66(2), 99–104 (2011)
X. Mao, A.A. Bol’shakov, D.L. Perry, O. Sorkhabi, R.E. Russo, Laser ablation molecular isotopic spectrometry: parameter influence on boron isotope measurements. Spectrochim. Acta Part B 66(8), 604–609 (2011)
A.A. Bol’shakov, X. Mao, J.J. González, R.E. Russo, Laser ablation molecular isotopic spectrometry (LAMIS): current state of the art. J. Anal. At. Spectrom. 31(1), 119–134 (2016)
R.S. Mulliken, The isotope effect in band spectra, II: the spectrum of boron monoxide. Phys. Rev. 25(3), 259 (1925)
X. Mao, G.C.-Y. Chan, I. Choi, V. Zorba, R.E. Russo, Combination of atomic lines and molecular bands for uranium optical isotopic analysis in laser induced plasma spectrometry. J. Radioanal. Nucl. Chem. 312(1), 121–131 (2017)
G.C.-Y. Chan, X. Mao, L.R. Martin, L.D. Trowbridge, R.E. Russo, Direct uranium enrichment assay in gaseous uranium hexafluoride with laser induced breakdown spectroscopy. J. Radioanal. Nucl. Chem. 331(3), 1409–1421 (2022)
Acknowledgements
There are many students, post docs, scientist, all friends and colleagues throughout the world who have contributed to my research on laser ablation. My 38-year position at the Lawrence Berkeley National Laboratory and 19+ year position at Applied Spectra have allowed me to work with talented people to explore and develop this interest.
Funding
Funding at LBNL has primarily been through the Department of Energy who recognized the potential of laser ablation for government and dual-use industrial applications. Applied Spectra has benefitted from the USA SBIR funding, a source of funding that significantly assists small businesses start-ups. I sincerely thank the editorial staff of Applied Physics A for awarding me the 2022 Julius Springer Prize in Applied Physics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Russo, R.E. Laser ablation research and development: 60 years strong. Appl. Phys. A 129, 168 (2023). https://doi.org/10.1007/s00339-023-06425-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06425-3