Abstract
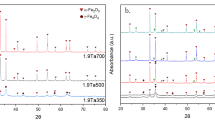
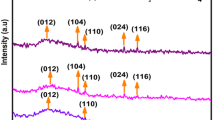
Hematite has been applied for many fields because of its excellent characteristics. It shows various magnetic characteristics such as weak-ferromagnetic, anti-ferromagnetic and superparamagnetic characters. Doping to hematite with other transition metal is one method to maintain one of magnetic properties. Nb5+ was used as a dopant for hematite to know the influence of pentavalent dopant toward the structure and characteristics of hematite. PXRD showed that Nb was incorporated to hematite lattice, while at higher calcination temperature Nb was excluded to the surface forming FeNbO4. The sample calcined at 600 °C mainly showed a hematite structure, but it was attracted to magnet. This is maybe because of ferrimagnetic interaction due to the introduction of Nb. The attraction to magnet became weak for the sample calcined at 700 °C, maybe due to the exclusion of Nb as FeNbO4. By exclusion of Nb, ferrimagnetic character changed to pure weak-ferromagnetism. Mӧssbauer spectrum measurement at 78 K (− 195 °C) showed that two sites in hematite crystal structure for sample calcined at 700 °C. The Mӧssbauer parameters showed that the weak-ferromagnetism partially exists even at lower temperature. Nb-doped (≥ 3.8 at. %) hematite calcined at 600 °C showed weak-ferromagnetism both at 78 and 298 K.







Similar content being viewed by others
References
M. Tadic, M. Panjan, V. Damnjanovic, I. Milosevic, Magnetic properties of hematite (α-Fe2O3) nanoparticles prepared by hydrothermal synthesis method. Appl Surf Sci 320, 183–187 (2014). https://doi.org/10.1016/j.apsusc.2014.08.193
K.A. Hinds, J.M. Hill, E.M. Shapiro et al., Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood 102, 867–872 (2003). https://doi.org/10.1182/blood-2002-12-3669
L. Huo, W. Li, L. Lu et al., Preparation, structure, and properties of three-dimensional ordered α-Fe2O3 nanoparticulate film. Chem Mater 12, 790–794 (2000). https://doi.org/10.1021/cm990690
W. Wu, C. Jiang, V.A.L. Roy, Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 7, 38–58 (2015). https://doi.org/10.1039/c4nr04244a
R.M. Cornell, U. Schwertmann, The iron oxides: structure, properties, reactions occurances and uses, 2nd edn. (Wiley-VCH, Weinheim, 2003)
R. Suresh, R. Prabu, A. Vijayaraj et al., Facile synthesis of cobalt doped hematite nanospheres: magnetic and their electrochemical sensing properties. Mater Chem Phys 134, 590–596 (2012). https://doi.org/10.1016/j.matchemphys.2012.03.034
A.S. Teja, P.Y. Koh, Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog Cryst Growth Charact Mater 55, 22–45 (2009). https://doi.org/10.1016/j.pcrysgrow.2008.08.003
C.S. Suman, A. Kumar, P. Kumar, Zn doped α-Fe2O3: an efficient material for UV driven photocatalysis and electrical conductivity. Crystals 10, 273 (2020). https://doi.org/10.3390/cryst10040273
C.D. Powell, A.W. Lounsbury, Z.S. Fishman et al., Nano-structural effects on Hematite (α-Fe2O3) nanoparticle radiofrequency heating. Nano Converg 81(8), 1–9 (2021). https://doi.org/10.1186/S40580-021-00258-7
M. Tadić, N. Čitaković, M. Panjan et al., Synthesis, morphology, microstructure and magnetic properties of hematite submicron particles. J Alloys Compd 509, 7639–7644 (2011). https://doi.org/10.1016/J.JALLCOM.2011.04.117
X. Xie, H. Yang, F. Zhang et al., Synthesis of hollow microspheres constructed with α-Fe2O3 nanorods and their photocatalytic and magnetic properties. J Alloys Compd 477, 90–99 (2009). https://doi.org/10.1016/J.JALLCOM.2008.10.161
M. Tadić, D. Marković, V. Spasojević et al., Synthesis and magnetic properties of concentrated α-Fe2O3 nanoparticles in a silica matrix. J Alloys Compd 441, 291–296 (2007). https://doi.org/10.1016/J.JALLCOM.2006.09.099
J. Lian, X. Duan, J. Ma et al., Hematite (α-Fe2O3) with various morphologies: ionic liquid-assisted synthesis, formation mechanism, and properties. ACS Nano 3, 3749–3761 (2009). https://doi.org/10.1021/NN900941E/SUPPL_FILE/NN900941E_SI_001.PDF
V. Panchal, U. Bhandarkar, M. Neergat, K.G. Suresh, Controlling magnetic properties of iron oxide nanoparticles using post-synthesis thermal treatment. Appl Phys A Mater Sci Process 114, 537–544 (2014). https://doi.org/10.1007/S00339-013-7610-X/FIGURES/6
S.T. Lin, Magnetic properties of hematite single crystals. I. Magnetization isotherm, antiferromagnetic susceptibility, and weak ferromagnetism of a natural crystal. Phys Rev 116, 1447–1452 (1959)
A. Aharoni, E.H. Frei, M. Schieber, Curie point and origin of weak ferromagnetism in hematite. Phys Rev 127, 439–441 (1962). https://doi.org/10.1103/PhysRev.127.439
D.W. Strangway, B.E. McMahon, R.M. Honea, E.E. Larson, Superparamagnetism in hematite. Earth Planet Sci Lett 2, 367–371 (1967). https://doi.org/10.1016/0012-821X(67)90158-6
Ö. Özdemir, D.J. Dunlop, T.S. Berquó, Morin transition in hematite: size dependence and thermal hysteresis. Geochem Geophys Geosyst (2008). https://doi.org/10.1029/2008GC002110/FORMAT/PDF
F. Bødker, M.F. Hansen, C.B. Koch et al., Magnetic properties of hematite nanoparticles. Phys Rev B 61, 6826–6838 (2000)
J.Z. Liu, Morin transition in hematite doped with iridium ions. J Magn Magn Mater 54–57, 901–902 (1986). https://doi.org/10.1016/0304-8853(86)90305-7
S. Shen, P. Guo, D.A. Wheeler et al., Physical and photoelectrochemical properties of Zr-doped hematite nanorod arrays. Nanoscale 5, 9867 (2013). https://doi.org/10.1039/c3nr03245k
R. Satheesh, K. Vignesh, A. Suganthi, M. Rajarajan, Visible light responsive photocatalytic applications of transition metal (M = Cu, Ni and Co) doped α-Fe2O3 nanoparticles. J Environ Chem Eng 2, 1956–1968 (2014). https://doi.org/10.1016/J.JECE.2014.08.016
A. Annamalai, P.S. Shinde, T.H. Jeon et al., Fabrication of superior α-Fe2O3 nanorod photoanodes through ex-situ Sn-doping for solar water splitting. Sol Energy Mater Sol Cells 144, 247–255 (2016). https://doi.org/10.1016/J.SOLMAT.2015.09.016
C. Sanchez, M. Hendewerk, K.D. Sieber, G.A. Somorjai, Synthesis, bulk, and surface characterization of niobium-doped Fe2O3 single crystals. J Solid State Chem 61, 47–55 (1986). https://doi.org/10.1016/0022-4596(86)90005-8
A. Annamalai, R. Sandströ, E. Gracia-Espino et al., Influence of Sb5+ as a double donor on Hematite (Fe3+) photoanodes for surface-enhanced photoelectrochemical water oxidation. ACS Appl Mater Interfaces 10, 16467–16473 (2018). https://doi.org/10.1021/acsami.8b02147
A.E. Gash, T.M. Tillotson, J.H. Satcher et al., Use of epoxides in the sol-gel synthesis of porous iron(III) oxide monoliths from Fe(III) salts. Chem Mater 13, 999–1007 (2001). https://doi.org/10.1021/cm0007611
M. Ristić, S. Popović, S. Musić, Sol–gel synthesis and characterization of Nb2O5 powders. Mater Lett 58, 2658–2663 (2004). https://doi.org/10.1016/J.MATLET.2004.03.041
I.L.O. Brasileiro, V.S. Madeira, C.P. De Souza et al., Development of α-Fe2O3/Nb2O5 photocatalysts by a Pechini sol-gel route: structural, morphological and optical influence. Mater Res Express 6, 15043 (2019). https://doi.org/10.1088/2053-1591/aae8cb
A.C. Silva, D.Q.L. Oliveira, L.C.A. Oliveira et al., Nb-containing hematites Fe2−xNbxO3: the role of Nb5+ on the reactivity in presence of the H2O2 or ultraviolet light. Appl Catal A Gen 357, 79–84 (2009). https://doi.org/10.1016/J.APCATA.2009.01.014
J. Jacob, M. Abdul Khadar, VSM and Mössbauer study of nanostructured hematite. J Magn Magn Mater 322, 614–621 (2010). https://doi.org/10.1016/j.jmmm.2009.10.025
S. Musić, I. Czakó-Nagy, I. Salaj-Obelić, N. Ljubešić, Formation of α-Fe2O3 particles in aqueous medium and their properties. Mater Lett 32, 301–305 (1997). https://doi.org/10.1016/S0167-577X(97)00051-7
D.M. Adams, Inorganic solids: an introduction to concept in solid-state structural chemistry (Wiley, Toronto, 1974)
A. Grabias, T. Xu, M. Sorescu, Effect of niobium valence on the mechanochemical activation of niobium oxides–hematite magnetic ceramic nanoparticles. Ceram Int 39, 5343–5357 (2013). https://doi.org/10.1016/J.CERAMINT.2012.12.040
U. Schwertmann, R.W. Fitzpatrick, R.M. Taylor, D.G. Lewis, Influence of aluminum on iron oxides part II. Preparation and properties of Al-substituted hematites. Clays Clay Miner 27, 105–112 (1979). https://doi.org/10.1346/CCMN.1979.0270205
L. Pauling, S.B. Hendricks, The crystal structure of hematite and corundum. J Am Chem Soc 47, 781–790 (1925)
T.J. Smart, M. Chen, A.C. Grieder et al., The critical role of synthesis conditions on small polaron carrier concentrations in hematite—a first-principles study. J Appl Phys 130, 245705 (2021). https://doi.org/10.1063/5.0074698
A.J. Bosman, H.J. van Daal, Small-polaron versus band conduction in some transition-metal oxides. Adv Phys 19, 1–117 (1970). https://doi.org/10.1080/00018737000101071
E. De Grave, L.H. Bowen, R. Vochten, R.E. Vandenberghe, The effect of crystallinity and Al substitution on the magnetic structure and morin transition in hematite. J Magn Magn Mater 72, 141–151 (1988). https://doi.org/10.1016/0304-8853(88)90182-5
S. Krehula, M. Ristić, M. Reissner et al., Synthesis and properties of indium-doped hematite. J Alloys Compd 695, 1900–1907 (2017). https://doi.org/10.1016/j.jallcom.2016.11.022
E. De Grave, L.H. Bowen, S.B. Weed, Mössbauer study of aluminum-substituted hematites. J Magn Magn Mater 27, 98–108 (1982). https://doi.org/10.1016/0304-8853(82)90288-8
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, H., Nakashima, S. 57Fe Mössbauer spectroscopic study on the magnetic structure of niobium-doped hematite. Appl. Phys. A 128, 564 (2022). https://doi.org/10.1007/s00339-022-05691-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-05691-x