Abstract
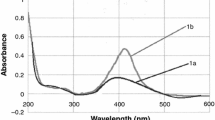
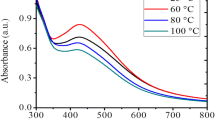
In this study, sericin extracted from Bombyx mori silk cocoons was integrated into the well-known Tollens’ method for synthesizing Ag-NPs. Sericin successfully acted as a stabilizer while silver amine complex [Ag(NH3)2]+ was reduced by maltose. As a result, silver nanoparticles with high stability are formed. Possible functional groups related to the stabilization of NPs were investigated by Fourier-transforms infrared spectroscopy (FT-IR). Ag-Ser NPs were characterized using particle size measurements based on dynamic light scattering (DLS) and transmission electron microscopy (TEM). According to the characterization investigations, Ag-Ser NPs have characteristic (111) face-centered cubic (FFC) plane and were spherical in shape with a narrow size distribution of 20.23 ± 6.25 nm. Overall, the sericin-modified Tollens’ method for synthesizing Ag-NPs offers a simple and non-toxic production method to form nanoparticles. Colloidal stability of nanoparticles displays an essential role since their enhanced nano-properties can be diminished by an increase in size due to aggregation and agglomeration. Therefore, the effect of pH and electrolyte concentration on particle stability was investigated through the surface charge of Ag-Ser NPs using a Zeta-potential analyzer and change in absorption spectra by UV–Vis Spectroscopy. Results obtained from this study propose a potential synthesis route for Ag NP synthesis and may extend the applicability of silver nanoparticles in biotechnological researches as aseptic and therapeutic usages.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, M.S., upon reasonable request.
Abbreviations
- Ag-Ser NPs:
-
Sericin stabilized silver nanoparticles
- FT-IR spectroscopy:
-
Fourier-transforms infrared spectroscopy
- DLS:
-
Dynamic light scattering
- TEM:
-
Transmission electron microscope
- HRTEM:
-
High-resolution transmission electron microscope image
- SAED:
-
Selected area (electron) diffraction
References
C. N. R. Rao, A. Müller, ve A. K. Cheetham, The chemistry of nanomaterials: synthesis, properties and applications, 2 Volumes. 2004. Erişim: 22 Şubat 2022. [Çevrimiçi]. Erişim adresi: https://ui.adsabs.harvard.edu/abs/2004cnsp.book.....R
P.M. Mendes, Cellular nanotechnology: making biological interfaces smarter. Chem. Soc. Rev. 42(24), 9207–9218 (2013). https://doi.org/10.1039/C3CS60198F
T.M. Tolaymat, A.M. El Badawy, A. Genaidy, K.G. Scheckel, T.P. Luxton, M. Suidan, An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 408(5), 999–1006 (2010). https://doi.org/10.1016/j.scitotenv.2009.11.003
D.M. Eby, N.M. Schaeublin, K.E. Farrington, S.M. Hussain, G.R. Johnson, Lysozyme catalyzes the formation of antimicrobial silver nanoparticles. ACS Nano 3(4), 984–994 (2009). https://doi.org/10.1021/nn900079e
D.M. Mitrano, E. Rimmele, A. Wichser, R. Erni, M. Height, B. Nowack, Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano 8(7), 7208–7219 (2014). https://doi.org/10.1021/nn502228w
K. Jadhav, Phytosynthesis of silver nanoparticles: characterization, biocompatibility studies, and anticancer activity. ACS Biomater. Sci. Eng. 4(3), 892–899 (2018). https://doi.org/10.1021/acsbiomaterials.7b00707
S.P. Deshmukh, S.M. Patil, S.B. Mullani, S.D. Delekar, Silver nanoparticles as an effective disinfectant: a review. Mater. Sci. Eng. C 97, 954–965 (2019). https://doi.org/10.1016/j.msec.2018.12.102
T. Maneerung, S. Tokura, R. Rujiravanit, Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 72(1), 43–51 (2008). https://doi.org/10.1016/j.carbpol.2007.07.025
P. Prasher, Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloid Interface Sci. Commun. 35, 100244 (2020). https://doi.org/10.1016/j.colcom.2020.100244
M. Sivera, Silver nanoparticles modified by gelatin with extraordinary pH stability and long-term antibacterial activity. PLoS One 9(8), e103675 (2014). https://doi.org/10.1371/journal.pone.0103675
H. He, In situ green synthesis and characterization of sericin-silver nanoparticle composite with effective antibacterial activity and good biocompatibility. Mater. Sci. Eng. C 80, 509–516 (2017). https://doi.org/10.1016/j.msec.2017.06.015
H. Huang, X. Yang, Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr. Res. 339(15), 2627–2631 (2004). https://doi.org/10.1016/j.carres.2004.08.005
A. de Lima, P.S. da Silva, A. Spinelli, Chitosan-stabilized silver nanoparticles for voltammetric detection of nitrocompounds. Sens. Actuators B Chem. 196, 39–45 (2014). https://doi.org/10.1016/j.snb.2014.02.005
M. Darroudi, M.B. Ahmad, A.H. Abdullah, N.A. Ibrahim, Green synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticles. Int. J. Nanomed. 6, 569–574 (2011). https://doi.org/10.2147/IJN.S16867
N.M. Zain, A.G.F. Stapley, G. Shama, Green synthesis of silver and copper nanoparticles using ascorbic acid and chitosan for antimicrobial applications. Carbohydr. Polym. 112, 195–202 (2014). https://doi.org/10.1016/j.carbpol.2014.05.081
S. Mohan, Completely green synthesis of dextrose reduced silver nanoparticles, its antimicrobial and sensing properties. Carbohydr. Polym. 106, 469–474 (2014). https://doi.org/10.1016/j.carbpol.2014.01.008
L.K.H. Rocha, Sericin from Bombyx mori cocoons. Part I: extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 61, 163–177 (2017). https://doi.org/10.1016/j.procbio.2017.06.019
O. Akturk, A. Tezcaner, H. Bilgili, M.S. Deveci, M.R. Gecit, D. Keskin, Evaluation of sericin/collagen membranes as prospective wound dressing biomaterial. J. Biosci. Bioeng. 112(3), 279–288 (2011). https://doi.org/10.1016/j.jbiosc.2011.05.014
Y. Yin, Z.-Y. Li, Z. Zhong, B. Gates, Y. Xia, S. Venkateswaran, Synthesis and characterization of stable aqueous dispersions of silver nanoparticles through the Tollens process. J. Mater. Chem. 12(3), 522–527 (2002). https://doi.org/10.1039/B107469E
L. Kvítek, R. Prucek, A. Panáček, R. Novotný, J. Hrbáč, R. Zbořil, The influence of complexing agent concentration on particle size in the process of SERS active silver colloid synthesis. J. Mater. Chem. 15(10), 1099–1105 (2005). https://doi.org/10.1039/B417007E
P. Aramwit, S. Kanokpanont, T. Nakpheng, T. Srichana, The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 11(5), 2200–2211 (2010). https://doi.org/10.3390/ijms11052200
H. Oh, J.Y. Lee, M.K. Kim, I.C. Um, K.H. Lee, Refining hot-water extracted silk sericin by ethanol-induced precipitation. Int. J. Biol. Macromol. 48(1), 32–37 (2011). https://doi.org/10.1016/j.ijbiomac.2010.09.008
H. Teramoto, M. Miyazawa, Molecular orientation behavior of silk sericin film as revealed by ATR infrared spectroscopy. Biomacromol 6(4), 2049–2057 (2005). https://doi.org/10.1021/bm0500547
D.C. CastrillónMartínez, C.L. Zuluaga, A. Restrepo-Osorio, C. Álvarez-López, Characterization of sericin obtained from cocoons and silk yarns. Proced. Eng. 200, 377–383 (2017). https://doi.org/10.1016/j.proeng.2017.07.053
A.-T. Le, Green synthesis of finely-dispersed highly bactericidal silver nanoparticles via modified Tollens technique. Curr. Appl. Phys. 10(3), 910–916 (2010). https://doi.org/10.1016/j.cap.2009.10.021
A. Panáček, Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 110(33), 16248–16253 (2006). https://doi.org/10.1021/jp063826h
S. Sangsuk, Preparation of high surface area silver powder via Tollens process under sonication. Mater. Lett. 64(6), 775–777 (2010). https://doi.org/10.1016/j.matlet.2010.01.013
G.A. Martínez-Castañón, N. Niño-Martínez, F. Martínez-Gutierrez, J.R. Martínez-Mendoza, F. Ruiz, Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 10(8), 1343–1348 (2008). https://doi.org/10.1007/s11051-008-9428-6
M. Darroudi, M.B. Ahmad, A.K. Zak, R. Zamiri, M. Hakimi, Fabrication and Characterization of gelatin stabilized silver nanoparticles under UV-light. Int. J. Mol. Sci. 12(9), 9 (2011). https://doi.org/10.3390/ijms12096346
Y.M. Mohan, K.M. Raju, K. Sambasivudu, S. Singh, B. Sreedhar, Preparation of acacia-stabilized silver nanoparticles: a green approach. J. Appl. Polym. Sci. 106(5), 3375–3381 (2007). https://doi.org/10.1002/app.26979
M. Chen, Y.-G. Feng, X. Wang, T.-C. Li, J.-Y. Zhang, D.-J. Qian, Silver nanoparticles capped by oleylamine: formation, growth, and self-organization. Langmuir 23(10), 5296–5304 (2007). https://doi.org/10.1021/la700553d
D. Philip, Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low-Dimens. Syst. Nanostruct. 42(5), 1417–1424 (2010). https://doi.org/10.1016/j.physe.2009.11.081
S. Shankar, L. Jaiswal, R.S.L. Aparna, R.G.S.V. Prasad, Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Lett. 137, 75–78 (2014). https://doi.org/10.1016/j.matlet.2014.08.122
E.J. Guidelli, A.P. Ramos, M.E.D. Zaniquelli, O. Baffa, Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 82(1), 140–145 (2011). https://doi.org/10.1016/j.saa.2011.07.024
K. Jyoti, M. Baunthiyal, A. Singh, Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 9(3), 217–227 (2016). https://doi.org/10.1016/j.jrras.2015.10.002
J. Neunzehn, F. Draude, U. Golla-Schindler, H.F. Arlinghaus, H.-P. Wiesmann, Detection of protein coatings on nanoparticles surfaces by ToF-SIMS and advanced electron microscopy. Surf. Interface Anal. 45(9), 1340–1346 (2013). https://doi.org/10.1002/sia.5287
J.F.A. de Oliveira, M.B. Cardoso, Partial aggregation of silver nanoparticles induced by capping and reducing agents competition. Langmuir 30(17), 4879–4886 (2014). https://doi.org/10.1021/la403635c
T. Klar, M. Perner, S. Grosse, G. von Plessen, W. Spirkl, J. Feldmann, Surface-plasmon resonances in single metallic nanoparticles. Phys. Rev. Lett. 80(19), 4249–4252 (1998). https://doi.org/10.1103/PhysRevLett.80.4249
A.M. El Badawy, T.P. Luxton, R.G. Silva, K.G. Scheckel, M.T. Suidan, T.M. Tolaymat, Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 44(4), 1260–1266 (2010). https://doi.org/10.1021/es902240k
W. Liu, Q. Zhou, J. Liu, J. Fu, S. Liu, G. Jiang, Environmental and biological influences on the stability of silver nanoparticles. Chin. Sci. Bull. 56, 2009–2015 (2011). https://doi.org/10.1007/s11434-010-4332-8
I. Fernando, Y. Zhou, Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere 216, 297–305 (2019). https://doi.org/10.1016/j.chemosphere.2018.10.122
C. Levard, E.M. Hotze, G.V. Lowry, G.E. Brown, Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol. 46(13), 6900–6914 (2012). https://doi.org/10.1021/es2037405
G.K. Vertelov, Y.A. Krutyakov, O.V. Efremenkova, A.Y. Olenin, G.V. Lisichkin, A versatile synthesis of highly bactericidal Myramistin® stabilized silver nanoparticles. Nanotechnology 19(35), 355707 (2008). https://doi.org/10.1088/0957-4484/19/35/355707
F. Ahsan, T.M. Ansari, S. Usmani, P. Bagga, An insight on silk protein sericin: from processing to biomedical application. Drug Res. 68(6), 317–327 (2018). https://doi.org/10.1055/s-0043-121464
S.V. Sokolov, K. Tschulik, C. Batchelor-McAuley, K. Jurkschat, R.G. Compton, Reversible or not? Distinguishing agglomeration and aggregation at the nanoscale. Anal. Chem. 87(19), 10033–10039 (2015). https://doi.org/10.1021/acs.analchem.5b02639
S. Ashraf, Protein-mediated synthesis, pH-induced reversible agglomeration, toxicity and cellular interaction of silver nanoparticles. Colloids Surf. B Biointerfaces 102, 511–518 (2013). https://doi.org/10.1016/j.colsurfb.2012.09.032
I. Kim, B.-T. Lee, H.-A. Kim, K.-W. Kim, S.D. Kim, Y.-S. Hwang, Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere 143, 99–105 (2016). https://doi.org/10.1016/j.chemosphere.2015.06.046
A.J. Kora, R. Manjusha, J. Arunachalam, Superior bactericidal activity of SDS capped silver nanoparticles: synthesis and characterization. Mater. Sci. Eng. C 29(7), 2104–2109 (2009). https://doi.org/10.1016/j.msec.2009.04.010
J. Hedberg, M. Lundin, T. Lowe, E. Blomberg, S. Wold, I.O. Wallinder, Interactions between surfactants and silver nanoparticles of varying charge. J. Colloid Interface Sci. 369(1), 193–201 (2012). https://doi.org/10.1016/j.jcis.2011.12.004
X. Li, J.J. Lenhart, H.W. Walker, Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir ACS J. Surf. Colloids 26(22), 16690–16698 (2010). https://doi.org/10.1021/la101768n
L. Kvítek, Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 112(15), 5825–5834 (2008). https://doi.org/10.1021/jp711616v
Acknowledgements
We would like to thank Dr. Banu Taktak Karaca and Biruni University for valuable contributions including Bradford assay, Zeta-potential measurements. Additionally, our sincere gratitude to NANOSILVER Kimya Sanayii Ticaret A.Ş. (Chemical Commerce & Industry Co.) for financial support, Dr. Yonca Alkan Göksü for FT-IR measurements and Selçuk University (ILTEK) for TEM images.
Author information
Authors and Affiliations
Contributions
MS conceptualized the research, conducted the all the experiments, analyzed the experiment data regarding to characterization of nanoparticles and drafted the manuscript. BB helped both the experiments and preparing the manuscript. SM measured particle size of the samples. ST supervised and provided the necessary materials for nanoparticle synthesis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saraçoğlu, M., Bacınoğlu, M.B., Mertdinç, S. et al. Synthesis of sericin coated silver nanoparticles (Ag-Ser) by modified Tollens’ method and evaluation of colloidal stability. Appl. Phys. A 128, 424 (2022). https://doi.org/10.1007/s00339-022-05568-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-05568-z