Abstract
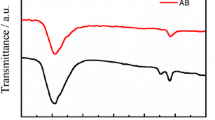
Poly (acrylonitrile) (PAN), acetylene black (AB) and graphene oxide (GO), as conductive constituents, were mixed with sulfur by solution-processing technique using dimethyl sulfoxide as a solvent. The physical properties of the as prepared composites were characterized by X-ray diffraction, Raman and scanning electron microscope analysis. The electrochemical property of S/GO composite material exhibits better cyclical stability. The first-cycle capacity obtained by S/GO composite was 937 mAh g−1 at 0.1 C with coulombic efficiency of 98% and good cycle ability around 813 mAh g−1 discharge capacity at the 50th cycle which is higher than of S/PAN and S/AB composite.
Graphic abstract







Similar content being viewed by others
References
B. Scrosati, J. Hassoun, Y.-K. Sun, Lithium-ion batteries. A look into the future. Energy Environ. Sci. 4, 3287–3295 (2011)
A.S. Ari, P. Bruce, B. Scrosati, J.M. Tarascon, W.V. Schalkwijk, Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 4, 366–377 (2005)
P.G. Bruce, S.A. Freunberger, L.J. Hardwick, J.M. Tarascon, Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012)
A. Manthiram, Y. Fu, Y. Su, Challenges and prospects of lithium-sulfur batteries. Chem. Res. 46, 1125–1134 (2012)
X. Ji, K.T. Lee, L.F. Nazar, A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 8, 500–506 (2009)
G. Radhika, R. Subadevi, K. Krishnaveni, W.R. Liu, M. Sivakumar, Synthesis and electrochemical performance of PEG-MnO2-sulfur composites cathode materials for lithium sulfur batteries. J. Nanosci. Nanotechnol. 18(1), 127–131 (2018)
P. Rajkumar, K. Diwakar, G. Radhika, K. Krishnaveni, R. Subadevi, M. Sivakumar, Effect of silicon dioxide in sulfur/carbon black composite as a cathode material for lithium sulfur batteries. Vacuum 161, 37–48 (2019)
K. Krishnaveni, R. Subadevi, M. Raja, T. PremKumar, M. Sivakumar, Sulfur/PAN/acetylene black composite prepared by a solution processing technique for lithium-sulfur batteries. J. Appl. Polym. Sci. 135, 46598 (2018)
J.Q. Li, C. Han, M.X. Jing, H. Yang, X.Q. Shen, S.B. Qin, Flakelike oxygen-deficient lithium vanadium oxides as a high ionic and electronic conductive cathode materials for high power Li-ion battery. Appl. Phys. A 124, 450 (2018)
A. Konarov, D. Gosselink, T.N.L. Doan, Y. Zhang, Y. Zhao, P. Chen, Simple, scalable, and economical preparation of sulfur-PAN composite cathodes for Li/S batteries. J. Power Sour. 259, 183–187 (2014)
L. Yin, J. Wang, F. Lin, J. Yang, Y. Nuli, Polyacrylonitrile/graphene composite as a precursor to a sulfur-based cathode material for high-rate rechargeable Li-S batteries. Energy Environ. Sci. 5, 6966–6972 (2012)
S.J. Rettig, J. Trotter, Refinement of the structure of orthorhombic sulfur, α-S8. Acta Cryst. C Cryst. Struct. Commun. 43, 2260–2262 (1987)
F. Tuz Johra, J.W. Lee, W.G. Jung, Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 20, 2883–2887 (2014)
J. Wang, J. Yang, C. Wan, K. Du, J. Xie, N. Xu, Sulfur composite cathode materials for rechargeable lithium batteries. Adv. Funct. Mater. 13, 487–492 (2003)
Y.V. Mikhaylik, J.R. Akridge, Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 151, A1969 (2004)
J. Li, K. Li, M. Li, D. Gosselink, Y. Zhang, P. Chen, Sulfur/polyacrylonitrile/graphene composite cathode for lithium batteries with excellent cyclability. J. Power Sources 252, 107–112 (2014)
K. Krishnaveni, R. Subadevi, G. Radhika, T. Premkumar, M. Raja, W.-R. Liu, M. Sivakumar, Carbon wrapping effect on sulfur/polyacrylonitrile composite cathode materials for lithium sulfur batteries. J. Nanosci. Nanotechnol. 18, 121–126 (2018)
A.T. Ward, Raman spectroscopy of sulfur, sulfur-selenium, and sulfur-arsenic mixtures. J. Phys. Chem. 72(12), 4133–4139 (1968)
P. Rajkumar, K. Diwakar, R. Subadevi, R.M. Gnanamuthu, M. Sivakumar, Sulfur cloaked with different carbonaceous materials for high performance lithium sulfur batteries. Curr. Appl. Phys. 19, 902–909 (2019)
J. Zhou, Y. Guo, C. Liang, J. Yang, J. Wang, Y. Nuli, Confining small sulfur molecules in peanut shell-derived microporous graphitic carbon for advanced lithium sulfur battery. Electrochim. Acta 273, 127–135 (2018)
T.H. Hwang, D.S. Jung, J.S. Kim, B.G. Kim, J.W. Choi, One-dimensional carbon–sulfur composite fibers for Na-S rechargeable batteries operating at room temperature. Nano Lett. 13, 4532–4538 (2013)
H. Sohn, M.L. Gordin, M. Regula, D.H. Kim, Y.S. Jung, J. Song, D. Wang, Porous spherical polyacrylonitrile–carbon nano composite with high loading of sulfur for lithium-sulfur batteries. J. Power Sour. 302, 70–78 (2016)
K. Krishnaveni, R. Subadevi, T. Premkumar, M. Raja, M. Sivakumar, Synthesis and characterization of graphene oxide capped sulfur/polyacrylonitrile composite cathode by simple heat treatment. J. Sulfur Chem. (2019). https://doi.org/10.1080/17415993.2019.1582655
X.M. He, W.H. Pu, J.G. Ren, L. Wang, J.L. Wang, C.Y. Jiang, C.R. Wan, Charge/discharge characteristics of sulfur composite cathode materials in rechargeable lithium batteries. Electrochim. Acta 52, 7372–7376 (2007)
Y.J. Choi, K.W. Kim, H.J. Ahn, J.H. Ahn, Improvement of cycle property of sulfur electrode for lithium/sulfur battery. J. Alloys Compd. 449, 313–316 (2008)
S.E. Cheon, K.S. Ko, J.H. Cho, S.W. Kim, E.Y. Chin, H.T. Kim, Rechargeable lithium sulfur battery. I Structural change of sulfur cathode during discharge and charge. J. Electrochem. Soc. 150, A796–A799 (2003)
S. Stankovich, D.A. Dikin, R.D. Piner, K.A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T. Nguyen, R.S. Ruoff, Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007)
T. Szabó, O. Berkesi, I. Dékány, DRIFT study of deuterium-exchanged graphite oxide. Carbon 43, 3186–3189 (2005)
A. Lerf, H. He, M. Forster, J. Klinowski, Structure of graphite oxide revisited. J. Phys. Chem. B 102, 4477–4482 (1998)
W.S. Hummers, R.E. Offeman, Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958)
L. Ji, M. Rao, H. Zheng, L. Zhang, Y. Li, W. Duan, J. Guo, E.J. Cairns, Y. Zhang, Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 133, 18522–18525 (2011)
W.G. Wang, X. Wang, L.Y. Tian, Y.L. Wang, S.H. Ye, In situ sulfur deposition route to obtain sulfur–carbon composite cathodes for lithium-sulfur batteries. J. Mater. Chem. A 2(12), 4316–4323 (2014)
Acknowledgements
All the authors acknowledge the financial support by DST-SERB, New Delhi under the Physical sciences, Grant sanctioned vide EMR/2016/006302. In addition, all the authors acknowledge for the financial support by BSR of University Grants Commission (UGC), New Delhi, India and Ministry of Human Resource Development RUSA-Phase 2.0 Grant sanctioned vide Lt.No.F-24-51/2014 U Policy (TN Multi Gen), Department of Education, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krishnaveni, K., Subadevi, R. & Sivakumar, M. A solution-processed binary composite as a cathode material in lithium–sulfur batteries. Appl. Phys. A 125, 469 (2019). https://doi.org/10.1007/s00339-019-2758-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-2758-7