Abstract
Coral reefs are exposed to various environmental stressors that cause bleaching events, whereby endosymbiotic microalgae (Symbiodiniaceae) disassociate from coral hosts. Bleached corals are compromised and face mortality. The combination of high-light exposure and elevated seawater temperature often lead to coral bleaching. The physiological properties of the Symbiodiniaceae within the coral tissues contribute to the thermal tolerance of the holobiont (the host and all its symbionts). The present study aimed to investigate the effects of light and temperature stress on four Symbiodiniaceae species from three genera with respect to photosynthetic oxygen production and consumption. Under control conditions, the species displayed predominantly low-to-moderate light requirements for photosynthesis with increased photoinhibition at higher photon flux rates. After 30 days of heat acclimation at 32 °C, maximum photosynthetic activity declined in Effrenium voratum, doubled in Fugacium kawagutii, and remained unchanged in Breviolum psygmophilum. In subsequent acute heating assays, species-specific effects on maximum photosynthetic activity were observed. Photosynthesis in all species declined across a temperature gradient between 25 and 39 °C in the acute heating assays; full inhibition occurred at 37 °C in B. psygmophilum and E. voratum and at 39 °C in B. aenigmaticum and F. kawagutii. In contrast, respiration remained largely constant in all species across temperatures. Our data point to species-specific photophysiological traits that lead to different thermal tolerances among Symbiodiniaceae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reef-building corals are unable to efficiently form reef structure (i.e., precipitate calcium carbonate) at low temperatures and are thus restricted to oligotrophic tropical regions between 30° north and south latitude (Kampmann 2002). Tropical coral reefs are hotspots of biodiversity and contribute multiple ecological and economic goods and services such as high productivity, complex habitat formation, food provisioning for numerous marine organisms, and coastal protection (Moberg and Folke 1999). In addition, tropical coral reefs rely on the mutualistic symbiosis between unicellular dinoflagellates (Symbiodiniaceae) and coral hosts (Decelle et al. 2018; LaJeunesse et al. 2018). Coral reefs are threatened by numerous natural and anthropogenic stressors, such as ocean warming and acidification, freshwater runoff, and water pollution, all of which can lead to coral bleaching (reviewed by van Oppen and Lough 2018). This phenomenon involves the dissociation of corals and their symbionts as a consequence of environmental stress. The longer this situation persists, the more likely it is that the corals will die. Coral bleaching has been observed multiple times on a global scale over the last decades (Hughes et al. 2017; Roth 2014). According to the latest Status Report of Coral Reefs of the World (Souter et al. 2021), there was a progressive loss of about 14% of the coral from the world’s reefs between 2009 and 2018, and as the main stressor a rapid rise in sea surface temperature due to marine heatwaves. The tropical coral-algal symbiosis typically exists within a narrow temperature range close to its upper thermal limit, and thus even small temperature anomalies of just 1–2 °C above mean local summer maxima are sufficient to cause coral bleaching (Lesser 2011; Rädecker et al. 2023).
Absorbed sunlight is essential for photosynthesis as it drives photochemical reactions, but the strength of light determines whether Symbiodiniaceae species are stressed or not (Roth 2014). Due to the dynamic nature of solar radiation, the photosynthetic machinery is often confronted with higher light levels than can be processed through photochemistry, and hence excess absorbed excitation energy must be removed by other pathways to minimize photooxidative damage (Müller et al. 2001; Niyogi 1999). These include reemission as chlorophyll fluorescence, dissipation as heat (non-photochemical quenching) or decay via the chlorophyll triplet state in which reactive oxygen species (ROS) are produced (Asada 1999; Müller et al. 2001). ROS often cause photooxidative damage to proteins and other biomolecules of the photosynthetic system and are inevitably produced during photosynthesis (Niyogi 1999). Consequently, the photosynthetic apparatus is constantly repairing itself from photodamage (Niyogi 1999). If the rate of damage exceeds the rate of repair, there will be a decline in photosynthetic performance (Roth 2014). Nevertheless, the photosynthetic apparatus is known as a plastic and flexible molecular machine, and thus capable of acclimating to dynamic and changing light conditions (Falkowski and Raven 2007), such as those on a coral reef.
The algal endosymbionts that form mutualisms with corals belong to the family Symbiodiniaceae (Dinophyceae, Alveolata). Though originally classified as a single species due to morphological homogeneity (Freudenthal 1962), the application of various molecular markers has revealed a genetically diverse group of dinoflagellates, which were assigned to multiple phylogenetic clades (e.g., “Clade A,” “Clade B,” etc.) (Rowan and Powers 1991). Most of these “Clades” were recently elevated to genera within the family Symbiodiniaceae (LaJeunesse et al. 2018, 2022; Nitschke et al. 2020; Pochon and LaJeunesse 2021). Species within each genus exhibit different ecophysiological traits, such as levels of tolerance to solar radiation (Suggett et al. 2015) and temperature fluctuations (Swain et al. 2017). The underlying mechanisms for this functional diversity are not fully understood, but can be, at least partially, explained by molecular and biochemical processes of the photosynthetic apparatus and other modes of resource acquisition (Takahashi et al. 2008; Suggett et al. 2017).
Based on studies of cultured algae, the effects of elevated temperature on members of the Symbiodiniaceae are not uniform (Lesser 1996; Karim et al. 2015; Klueter et al. 2017). For example, Karim et al. (2015) revealed that growth and photochemical efficiencies (Fv/Fm) of photosystem II (PSII) remained unchanged between 25 and 30 °C in various Symbiodiniaceae lineages. Both physiological traits strongly declined at 33 °C in thermally sensitive taxa, but not in thermally tolerant ones. In contrast, at temperatures > 33 °C, the thermal tolerance of all investigated species was exceeded. Other studies confirmed a similar pattern, showing that different Symbiodiniaceae cultures exhibit specific tolerances with respect to light and temperature gradients (Robison and Warner 2006; Diaz-Almeyda et al. 2017). Such variation is not restricted to the free-living condition; light and temperature tolerance are species-specific traits among Symbiodiniaceae residing within coral hosts (Berkelmans and van Oppen 2006; Sampayo et al. 2008; van Oppen et al. 2009). Ultimately, the interaction between host and symbiont genotype dictates various aspects of holobiont physiology (Parkinson and Baums 2014).
The main goal of the present study was to evaluate variation in the light requirements and thermal tolerances of several different Symbiodiniaceae species from multiple genera. Breviolum psygmophilum is a cold-tolerant symbiotic species from the Western Atlantic Ocean, whereas B. aenigmaticum is a closely-related but likely free-living Symbiodiniaceae with unknown thermal tolerance (Bayliss et al. 2019; Parkinson et al. 2015; Thornhill et al. 2008). Effrenium voratum is a free-living, highly motile species distributed globally in temperate waters (Jeong et al. 2014; Lee et al. 2016). Fugacium kawagutii is also a free-living species mostly found in the Pacific Ocean (LaJeunesse et al. 2018). These four Symbiodiniaceae were comparatively investigated under controlled experimental conditions (i.e., standard maintenance at 26 °C followed by 30 days incubation at 32 °C). Photosynthetic and respiratory responses were evaluated to address light requirements, thermal tolerance, and acclimation capacity.
Material and methods
Culture and cultivation
In this study, we evaluated the photophysiological properties and thermal tolerance of four isoclonal cultures representing four Symbiodiniaceae species: Breviolum aenigmaticum (strain 04–180), B. psygmophilum (strain Pur.P.flex), Effrenium voratum (strain CCMP 421), and Fugacium kawagutii (strain MV) (Tab.1).The cultures were maintained in marine sterilized North Sea water (Helgoland, 30.7 SA (absolute salinity)) enriched with 30 mL L−1 Guillard’s f/2 medium (Guillard and Ryther 1962) which provides trace metals and vitamins. For each species, three replicate 50 mL Erlenmeyer flasks were established which were then covered with foam stoppers and aluminum foil. These glass vessels were kept in a water bath to ensure a stable temperature for cultivation at 26 °C (± 0.8 °C, Jumo, TDA-300, Fulda, Germany) by establishing a constant water flow (Profi Cool, UKT 300-1,National Lab GmbH, Mölln, Germany). Using a 36W neon tube (Osram Lumilux® De Luxe L36W/840 Cool Light, Osram, Munich, Germany), an average photon flux density of 74 µmol photons m−2 s−1 was applied in combination with a light–dark rhythm of 12 h:12 h. All cultures were acclimated at 26 °C for 2–3 months and the media regularly changed to grow sufficient biomass (5–10 mg chlorophyll a L−1) for the experiments. Media composition was identical to that mentioned before (Table 1).
Temperature treatments
For each culture, after sufficient biomass was produced as indicated by the algal cultures reaching log phase (see above), each replicate flask was split into two equal sub-volumes. One sub-volume was maintained at 26 °C for an additional 30 days (control treatment) while in parallel, the other sub-volume was maintained in a separate water bath at 32 °C for 30 days (elevated temperature treatment) under the same light conditions.
Fixed temperature, variable light experiments
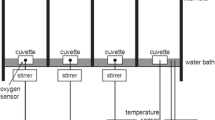
For each species and treatment, photosynthesis-irradiance curves (PE curves) were recorded employing a custom-built PE box (Prelle et al. 2022, Supplemental Fig. 1) which determines oxygen production per chlorophyll a as a function of increasing photon flux densities (PFDs). PE curves were generated in three separate water-surrounded oxygen electrode chambers (Hansatech Instruments, King’s Lynn, United Kingdom), each filled with 3 mL of culture media on top of a magnetic stirrer (Hansatech Instruments, King’s Lynn, United Kingdom). These chambers were temperature-controlled by connecting to a water supply (K10, Thermo Haake, Karlsruhe, Germany) and a thermostat (DC10, Thermo Haake, Karlsruhe, Germany) to ensure constant conditions during the measurements at 26 or 32 °C (± 0.1 °C). As external light sources, LEDs were applied (LUXEON Rebel1 LXML-PWN1-0100, neutral white, Phillips, Amsterdam), which were implemented in the PE box. To reduce the PFDs, neutral density filters were placed between each LED and the respective cuvette to generate a gradient of PFDs between 0 and 1,600 µmol photons m−2 s−1. The irradiance was measured directly inside the cuvettes using a small spherical light sensor (Light meter LI-250, LI-COR, Lincoln, USA). Each cuvette was equipped with an oxygen dipping probe DP sensor (optode) (PreSens Precision Sensing GmbH, Regensburg, Germany) and connected via a fiber optic to an oxygen transmitter (Oxy 4-Mini, PreSens Precision Sensing GmbH, Regensburg, Germany) in combination with the PreSens software OXY4v2_30 for measuring and calibration (two-point calibration, 0 and 100% oxygen saturation). To calibrate the oxygen dip probes, each cuvette was treated at 0 and 100% oxygen saturation at 20 °C, respectively. Oxygen saturation at 100% was performed by aeration of the culture media for 15 min. Sodium dithionite (Na2S2O4) was used to achieve oxygen free media. The chambers were tempered to the desired temperature (26 and 32 °C, respectively, for recording PE curves), and always 30 µL of sodium bicarbonate (NaHCO3, final concentration 2 mM) was added to each sample to avoid carbon deficiency (for details, see Prelle et al. 2022). After each PE curve, Symbiodiniaceae cell suspensions from each cuvette were filtered onto an individual Whatman GF/6 glass fiber filter (Ø 25 mm) for chlorophyll a determination as a reference parameter according to spectrophotometric assay of HELCOM (2015) which uses 96% ethanol (v/v) for pigment extraction. The photosynthetic model of Walsby (1997) was used for fitting and calculating different PE curve parameters:
where NPP is the net photosynthetic rate, NPPmax is the maximum photosynthetic rate at light-saturating irradiances (I), R is the respiration rate, α is the light utilization coefficient, and ß is the photoinhibition coefficient. The light saturation point (Ek) is calculated as NPPmax/α and the light compensation point (Ec) is calculated as − R/α.
Fixed light, variable temperature experiments
For each species and treatment, changes in photosynthesis and respiration rates were recorded as the Symbiodiniaceae responded to rising temperatures in the PE box following the approach of Russnak et al. (2021). These authors used the same batch of culture during the variable temperature experiment. Starting at 25 °C, a 20-min dark incubation phase was followed by a 10 min dark respiration phase and afterward by an additional 10 min photosynthesis phase in the light (340 µmol photons m−2 s−1, which typically stimulates maximal photosynthesis in Symbiodiniaceae; Falkowski and Raven 2007). After determining photosynthetic oxygen production, the temperature was increased by 2 °C within 3–4 min and a new incubation cycle (20 min incubation, 10 min respiration, and 10 min photosynthesis) was started after reaching the new temperature in the thermostat chamber. Finally, oxygen consumption in the dark and oxygen production in the light per unit time was related to the chlorophyll a concentration per sample as described above. From the data, the optimum temperatures for photosynthesis and respiration were calculated by applying the nonlinear model of Blanchard et al. (1996):
where Pmax (T) is the net photosynthetic rate, Pmax is the maximum value of Pmax, T is the temperature (°C), Tmax is the maximum temperature, Topt is the optimum temperature, and ß is a dimensionless parameter for sensitivity.
Statistical analysis
Data calculations were performed using Microsoft Office 365 Excel. To calculate the PE curves according to the Walsby model (Walsby 1997), the solver function was used to minimize the normalized deviation squares. The statistical analysis was performed using SPSS Statistics (version 27) and the program R (version 4.2.2). To calculate significance levels among all means, one-way ANOVA was used followed by a post hoc Tukey test. If the data did not fulfill the assumptions of variance homogeneity or normal distribution for one-way ANOVA, the Kruskal–Wallis test was applied. The significance level was set to α < 0.05 for all analyses.
Results
Light requirements for photosynthesis under ambient and elevated temperatures
Photosynthetic oxygen production in all four Symbiodiniaceae species at 26 °C (control treatment) revealed slightly different PI curves (Fig. 1). While Fugacium kawagutii showed no indication of photoinhibition up to 1,361 µmol photons m−2 s−1, Breviolum aenigmaticum and Effrenium voratum exhibited very small degrees of photoinhibition (ß = − 0.01 µmol O2 mg−1 Chl a h−1 (µmol photons m−2 s−1)−1) (Fig. 1, Table 2). In contrast, photosynthesis of Breviolum psygmophilum was moderately photoinhibited (ß = − 0.05 µmol O2 mg−1 Chl a h−1 (µmol photons m−2 s−1)−1) at the highest PFD (Fig. 1). In addition, the maximal oxygen production rate was similar in both Breviolum species and E. voratum (NPPmax range: 76.0–90.8 µmol O2 mg−1 Chl a h−1), and 2.5-fold lower in F. kawagutii (32.6 µmol O2 mg−1 Chl a h−1) (Fig. 1, Table 2). Among all species, the α-values ranged from 1.3 to 2.6 µmol O2 mg−1 Chl a h−1 (µmol photons m−2 s−1)−1, the Ek values from 40.4 to 78.5 µmol photons m−2 s−1, and that of EC were between 18.4 and 32.5 µmol photons m−2 s−1 (Table 2). All these data indicate generally low-to-moderate light requirements for photosynthesis at 26 °C, although some species-specific response patterns can be observed.
Photosynthesis and respiration rates (µmol O2 mg−1 Chl a h−1) as a function of increasing photon flux densities (µmol photons m−2 s.−1) of four Symbiodiniaceae species kept at 26 °C and at 32 °C for 30 days. The endosymbionts were kept in f/2 Baltic Sea water medium, 33SA, and oxygen changes were measured using optodes. Data represent mean values ± SD (n = 3), except for Breviolum aenigmaticum (n = 1, due to technical problems). Data points were fitted using the model of Walsby (1997)
Incubations of 30 days at 32 °C led to species-specific effects on the PE curve parameters of all investigated Symbiodiniaceae (Fig. 1, Table 2). While in B. psygmophilum NPPmax was unaffected, in E. voratum a significant reduction in NPPmax could be observed, dropping from 76.0 to 59.4 µmol O2 mg−1 Chl a h−1 with higher temperature (p < 0.05), and the opposite was the case in F. kawagutii, where NPPmax more than doubled under the higher temperature (from 32.6 to 70.4 µmol O2 mg−1 Chl a h−1; p < 0.05) (Table 2). In B. aenigmaticum, there also appeared to be a slight enhancement of NPPmax after treatment with 32 °C, but only one replicate could be measured due to a technical failure. While most PE curve parameters of the four species exhibited minor changes under increased temperatures, photoinhibition in B. psygmophilum was much less pronounced at 32 °C compared to 26 °C as reflected in a fivefold reduction in the ß-value at elevated temperature (− 0.05 to − 0.01 µmol O2 mg−1 Chl a h−1 (µmol photons m−2 s−1)−1; p < 0.05, Table 2).
Temperature effects on photosynthesis and respiration
Temperature had a strong effect on photosynthetic oxygen production and a much weaker influence on respiratory oxygen consumption in all four Symbiodiniaceae species (Fig. 2). Using cultures from the control treatment, the optimum temperature for photosynthesis was calculated using the Blanchard et al. (1996) model, resulting in optimum values ranging from 26.8 to 29.5 °C depending on the species (data not shown). With the same approach, the optimum temperature for respiration was calculated, and here a broader range was observed. While both Breviolum species showed a respiration optimum at 25 °C, E. voratum and F. kawagutii showed optima between 37 and 39 °C.
Photosynthetic oxygen production and respiratory consumption rates (µmol O2 mg−1 Chl a h−1) as function of increasing temperature (25–39 °C) at a saturating photon fluence density (PFD) of approx. 350 µmol photons m−2 s−1 in four Symbiodiniaceae species kept at 26 °C and after 30 days treatment at 32 °C. The endosymbionts were kept in f/2 Baltic Sea water medium, 33SA, and oxygen changes were measured using optodes. Data represent mean values ± SD (n = 3). Lowercase letters at photosynthesis and respiration indicate significantly means (p < 0.05; one-way ANOVA with post hoc Tukey-HSD test)
In B. psygmophilum and E. voratum photosynthesis was fully inhibited at 37 °C (Fig. 2). Photosynthesis in B. aenigmaticum and F. kawagutii was still measurable at 37 °C (c. 10–40% of the maximum), and fully inhibited at 39 °C (Fig. 2).In contrast, respiration essentially remained constant regardless of temperature (Fig. 2).
After 30 days of incubation at 32 °C, photosynthesis and respiration responses varied relative to counterparts in the control treatment for some species but not others (Fig. 2). In both Breviolum species NPPmax decreased > twofold and the maximum respiration rate declined 1.3–1.9 fold compared to the control treatment (Fig. 2).
Applying the model of Blanchard et al. (1996), we first calculated the optimum temperature for photosynthesis and respiration, followed by determination of the percentiles of < 20%, 20–80%, and > 80% (Fig. 3). This approach led to a visualization of the thermal limits of both physiological processes when reared at either 26 °C or 32 °C. Both Breviolum species lost photosynthetic capacity when reared at higher temperatures, while E. voratum and F. kawagutii were less affected. No species could photosynthesize when exposed to temperatures in excess of 37 °C, and all species maintained comparable respiration rates across a broad temperature range regardless of initial rearing temperature (Fig. 3).
Influence of temperature between 25 °C and 39 °C on photosynthetic oxygen production and respiratory oxygen consumption of four tested Symbiodiniaceae species using the fit of Blanchard et al. (1996). The black box represents area of highest photosynthesis more than > 80% percentile, dark gray symbols within 20 and 80% percentile, light gray symbols < 20% percentile, and white spaces no photosynthesis or respiratory signal. The data represent mean values (n = 3)
Discussion
Corals are susceptible to abiotic stressors such as increased solar radiation and heat waves, and the physiological plasticity and genetic diversity of the endosymbiotic microalgae are a key element explaining the different response patterns of their hosts (Berkelmans and van Oppen 2006; Sampayo et a. 2008; Baird et al. 2009; DeSalvo et al. 2010). Therefore, we grew four Symbiodiniaceae species under fixed light conditions and different temperatures, and evaluated photosynthesis and respiration activity to better understand physiological tolerance and plasticity.
Within a given temperature treatment, all Symbiodiniaceae species seem to exhibit similar patterns of photoacclimation when exposed to a range of light levels (Fig. 1). Symbiodiniaceae are capable of photoacclimation under changing photon flux rates (Iglesias-Prieto and Trench 1994; Roth 2014; Russnak et al. 2021), and our results align with this notion. In addition, Symbiodiniaceae increase chlorophyll and carotenoid concentrations in response to low-light conditions, thereby maximizing light absorption and utilization as well as improving photosynthetic efficiency (Anthony and Hoegh-Guldberg 2003). The conditions under which the algae were cultured are reflective of typical low-light levels within coral host tissue (Anthony et al. 2005), and were equivalent for all species, so it was assumed that chlorophyll levels remained constant within each species. However, because P:E data were normalized to chlorophyll content rather than cell counts, it is possible that certain differences in acclimation were masked (e.g., if pigment density changed with temperature). Future work should incorporate cell counts and verification of chlorophyll content per cell to increase confidence in species-specific patterns.
Our light experiment data are in agreement with those of Russnak et al. (2021) who investigated two genetically distinct genotypes of Breviolum psygmophilum (strains MAC HIAP and 1046) and reported Ek, Ec and respiration values similar to those measured in this study for B. psygmophilum (strain Pur.P.flex) under control conditions (70–80 µmol photons m−2 s−1). Furthermore, Russnak et al. (2021) examined the same isolate (genotype) of Effrenium voratum (strain CCMP 421), which yielded PE curve parameters comparable to those in the present investigation.
However, it is important to keep in mind that the physiology of cultured algae (in vitro) may not reflect their physiology when engaged in symbiosis (in hospite) (Maruyama and Weis 2021). Bhagooli and Hidaka (2003) studied the consequences of light stress on the maximum quantum yield (Fv/Fm) of PSII of different Symbiodiniaceae species in vitro and in hospite. They found that within host cells, symbiont Fv/Fm decreased significantly at high-light conditions (1000 µmol photons m−2 s−1), while in the respective isolated cultures, Fv/Fm declined precipitously at 70 µmol photons m−2 s−1 and higher, supporting the photoprotective role of host tissue. The coral host has a set of mechanisms to protect their endosymbionts against high-light conditions, while also optimizing their intracellular light environment depending on incident solar radiation (Lichtenberg et al. 2016). These mechanisms include, for example, the production of chromoproteins or other fluorescent pigments to quench harmful UV radiation, the formation of antioxidative enzymes, contraction and expansion of the tissue (gastrodermis) leading to modulation of intra-tissue light scattering, or the modification of the calcium carbonate skeleton to enhance light absorption (Lichtenberg et al. 2016; Roth 2014). Consequently, the coral host controls and regulates antioxidants, pigments, gene expression, behavior, and architectural levels over multiple timescales in response to changing light conditions to optimize the photobiological performance of the coral-algal symbiosis (Roth 2014). Further study of the performance of symbiotic Breviolum psygmophilum in hospite is warranted to better understand these protective mechanisms.
All four Symbiodiniaceae species exhibited optimum photosynthesis between 26.8 and 29.5 °C, and declining photosynthetic rates became evident with rising temperatures up to 35 °C (Fig. 2). While at 37 °C photosynthesis in B. aenigmaticum and F. kawagutii was still detectable, it was not in B. psygmophilum and E. voratum, and at 39 °C it was completely inhibited in all species. Gregoire et al. (2017) reported that their culture of B. psygmophilum (strain Mf11.05.01) was completely inhibited at just 33 °C, as was a culture of Symbiodinium tridacnidorum, whereas cultures of Breviolum minutum, Cladocopium infistulum, Durusdinium trenchii, and Fugacium kawagutii could still photosynthesize to varying degrees at this temperature. Russnak et al. (2021) comparatively investigated nine Symbiodiniaceae cultures (representing five species) and found that in four isolates (including E. voratum strain CCMP 421) photosynthesis was completely inhibited at 35 °C. The remaining five strains exhibited photosynthetic efficiencies between 20 and 80% of the maximum at 35 °C (Russnak et al. 2021), while at 40 °C photosynthesis was inhibited in all cultures.
The results from the present study point to clear species-specific and even strain-specific upper temperature thresholds for Symbiodiniaceae photosynthesis, typically in the range of 30 to 37 °C, and an absolute limit at 39 °C (Fig. 3). These thresholds are consistent with the 32–34 °C range typically reported for other cultured Symbiodiniceae (Iglesias-Prieto et al. 1992; Warner et al. 1996; Iglesias-Prieto and Trench 1994; Jones et al. 1998; Brown et al.1999). Given the thermal sensitivity of holobionts, shuffling or switching of Symbiodiniaceae communities in some corals after severe bleaching events may provide one of several mechanisms to acclimate (Huang et al. 2020). Although shifts in the dominant Symbiodiniaceae toward more thermotolerant species are commonly observed on the reef following bleaching, most novel associations do not persist long-term (Hume et al. 2020). Even if we consider that novel associations may persist under altered environmental regimes (i.e., if conditions don’t return to their previous state), our data argue that there is an upper thermal limit of algal symbionts that is close to the summer conditions in the warmest sea where corals exist: the Persian/Arabian Gulf (Hume et al 2016). These limitations must be considered in the current debate over active interventions, in particular regarding nature-based solutions (Buerger et al. 2020; Quigley et al. 2021; Voolstra et al. 2021). Under natural conditions tropical surface water temperatures typically range from 27 to 32 °C, but anomalies of up to 2.5 °C above the summer thermal maximum have been observed during strong El-Niño events (NOAA). Factoring in climate change, extremes are likely to increase to + 3.0 to 5.0 °C in the coming decades (IPCC 2023). Such temperature rise would reach the photosynthesis threshold of many symbiont species, with negative consequences for reef-building corals.
The conspicuous variation in Symbiodiniaceae thermal tolerance across cultures might be explained by an array of mechanisms. Enhanced temperatures can inhibit the repair mechanism of PSII, particularly the D1 proteins in the reaction centers, resulting in photoinhibition. This occurs when the damage to PSII exceeds its repair capacity, and symbiont strains capable of photosynthesis at temperatures above 32 °C likely have efficient thermoprotective mechanisms (Amario et al. 2023). These mechanisms may include the upregulation of various ROS scavenging proteins and molecular chaperones (Howells et al. 2012). For example, members of the Symbiodiniaceae synthesize heat shock protein 70 under thermal stress in order to repair and refold proteins (Ellison et al. 2017).
While photosynthesis exhibited a clear upper thermal limit for complete inhibition, respiration in all four species was mostly unaffected by temperature between 25 °C and 39 °C (Fig. 3). These data indicate that even under the highest temperature the endosymbionts were not dead because respiration was detectable. Such conspicuously distinct temperature requirements and tolerances for photosynthesis and respiration have previously been reported in closely-related Symbiodiniaceae (Gregoire et al. 2017; Russnak et al. 2021), as well as in other dinoflagellates (Lindström 1984), benthic diatoms (Prelle et al. 2022), and green algae (Karsten et al. 2014). It seems that in many microalgal taxa, respiration under low temperature is just detectable compared to photosynthesis, while the opposite is true for higher temperatures. This is not surprising, as key differences in both physiological processes exist. For example, the upstream mechanisms of photosynthesis are controlled mostly by light-related processes such as light absorption and energy transfer, whereas respiration is controlled almost entirely by temperature-dependent enzymatic reactions (Atkin and Tjoelker 2003). Nevertheless, the downstream process of carbon fixation involves various enzymes and hence might also be affected by temperature. In addition, photosynthesis is restricted to chloroplasts and respiration to mitochondria, and both organelles differ in their internal membrane systems and stability. The thylakoid structure of chloroplasts is much more vulnerable to light and temperature stress compared to mitochondrial cristae (Kirchoff 2014; Gounaris et al. 1984; Rurek 2014), which likely explains the greater functionality of respiration compared to photosynthesis under elevated temperatures. Under high temperature conditions photosynthesis is inhibited, while respiration becomes more and more essential to maintain the cellular energy demand (Slot et al. 2014).
Conclusion
In conclusion, we show species-specific physiological traits for Symbiodiniaceae cultures under controlled and simulated light and temperature conditions, which helped to identify light requirements and thermal tolerances of photosynthesis and respiration in four species. Our data confirm that photosynthesis and respiration responses are mostly uncoupled in these organisms. Further, respiration is typically less sensitive to temperature than photosynthesis. While results from culture-based studies cannot be directly transferred to the natural conditions of corals on the reef, where symbionts are buffered from the external environment by their hosts and colonies are exposed to more variable temperature fluctuations on different time scales, they nevertheless provide valuable insights into Symbiodiniaceae physiology.
Data availability
All data are presented in this study. The raw data are available on request by the corresponding author.
References
Amario M, Villela LB, Jardim-Messeder D, Silva-Lima AW, Rosado PM, de Moura RL, Sachetto-Martins G, Chaloub RM, Salomon PS (2023) Physiological response of Symbiodiniaceae to thermal stress: Reactive oxygen species, photosynthesis, and relative cell size. PLoS ONE 18:e0284717. https://doi.org/10.1371/journal.pone.0284717
Anthony KRN, Hoegh-Guldberg O (2003) Kinetics of photoacclimation in corals. Oecologia 134:23–31. https://doi.org/10.1007/s00442-002-1095-1
Anthony KRN, Hoogeboom MO, Connolly SR (2005) Adaptive variation in coral geometry and the optimization of inernal colony light climates. Funct Ecol 19:17–26. https://doi.org/10.1111/j.0269-8463.2005.00925.x
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639. https://doi.org/10.1146/annurev.arplant.50.1.601
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351. https://doi.org/10.1016/S1360-1385(03)00136-5
Baird AH, Bhagooli R, Ralph PJ, Takahashi S (2009) Coral bleaching: the role of the host. Trends Ecol Evol 24:16–20. https://doi.org/10.1016/j.tree.2008.09.005
Bayliss SLJ, Scott ZR, Coffroth MA, Horst CP (2019) Genetic variation in Breviolum antillogorgium, a coral reef symbiont, in response to temperature and nutrients. Ecol Evo 9:2803–2813. https://doi.org/10.1002/ece3.4959
Berkelmans R, Van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change. Proc R Soc Lond B 273:2305–2312. https://doi.org/10.1098/rspb.2006.3567
Bhagooli R, Hidaka M (2003) Comparison of stress susceptibility of in hospite and isolated zooxanthellae among five coral species. J Exp Mar Biol Ecol 291:181–197. https://doi.org/10.1016/S0022-0981(03)00121-7
Blanchard G, Guarini J, Richard P, Gros P, Mornet F (1996) Quantifying the short-term temperature effect on light-saturated photosynthesis of intertidal microphytobenthos. Mar Ecol Prog Ser 134:309–313. https://doi.org/10.3354/meps134309
Brown BE, Ambarsari I, Warner ME, Fitt WK, Dunne RP, Gibb SW et al (1999) Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: Evidence for photoinhibition and photoprotection. Coral Reefs 18:99–105. https://doi.org/10.1007/s003380050163
Buerger P, Alvarez-Roa C, Coppin CW, Pearce SL, Chakravarti LJ, Oakeshott JG, Edwards OR, van Oppen MJH (2020) Heat-evolved microalgal symbionts increase coral bleaching tolerance. Sci Adv 6:eaba2498. https://doi.org/10.1126/sciadv.aba2498
Decelle J, Carradec Q, Pochon X, Henry N, Romac S, Mahé F, Dunthorn M, Kourlaiev A, Voolstra CR, Wincker P, de Vargas C (2018) Worldwide occurrence and activity of the reef-building coral symbiont Symbiodinium in the open ocean. Curr Biol 28:3625-3633.e3. https://doi.org/10.1016/j.cub.2018.09.024
DeSalvo MK, Sunagawa S, Fisher PL, Voolstra CR, Iglesias-Prieto R, Medina M (2010) Coral host transcriptomic states are correlated with Symbiodinium genotypes. Mol Ecol 19:1174–1186. https://doi.org/10.1111/j.1365-294X.2010.04534.x
Díaz-Almeyda EM, Prada C, Ohdera AH, Moran H, Civitello DJ, Iglesias-Prieto R, Carlo TA, LaJeunesse TC Medina M (2017). Intraspecific and interspecific variation in thermotolerance and photoacclimation in Symbiodinium dinoflagellates. Proc Royal Soc B Biol Sci 6: 284:20171767. https://doi.org/10.1098/rspb.2017.1767
Ellison MA, Ferrier MD, Carney SL (2017) Salinity stress results in differential Hsp70 expression in the Exaiptasia pallida and Symbiodinium symbiosis. Mar Environ Res 132:63–67. https://doi.org/10.1016/j.marenvres.2017.10.006
Falkowski PG, Raven JA (2007) Aquatic Photosynthesis. Princeton University Press, New Jersey, p 484
Freudenthal HD (1962) Symbiodinium gen. nov. and Symbiodinium microadriaticum sp. nov., a zooxanthella: taxonomy, life cycle, and morphology. J Protozool 9:45–52. https://doi.org/10.1111/J.1550-7408.1962.TB02579.X
Gounaris K, Brain ARR, Quinn PJ, Williams WP (1984) Structural reorganisation of chloroplast thylakoid membranes in response to heat-stress. Biochim Biophys Acta-Bioenerg 766:198–208. https://doi.org/10.1016/0005-2728(84)90232-9
Grégoire V, Schmacka F, Coffroth MA, Karsten U (2017) Photophysiological and thermal tolerance of various genotypes of the coral endosymbiont Symbiodinium sp. (Dinophyceae). J Appl Phycol 29:1893–1905. https://doi.org/10.1007/s10811-017-1127-1
Guillard RRL, Ryther JH (1962) Studies or marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol 8: 229–239.
HELCOM (2015) Annex C-4. Phytoplankton chlorophyll a, in HELCOM Combine, (Helsinki: HELCOM), 257–263. Available online: https://helcom.fi/wp-content/uploads/2019/08/Guidelines-for-measuring-chlorophyll-a.pdf
Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, van Oppen MJH (2012) Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat Clim Change 2:116–120. https://doi.org/10.1038/nclimate1330
Huang YY, Carballo-Bolaños R, Kuo CY, Keshavmurthy S, Chen CA (2020) Leptoria phrygia in Southern Taiwan shuffles and switches symbionts to resist thermal-induced bleaching. Sci Rep 10:7808. https://doi.org/10.1038/s41598-020-64749-z
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, van de Leemput IA, Lough JM, Morrison TH, Palumbi SR, van Nes EH, Scheffer M (2017) Coral reefs in the Anthropocene. Nature 546:82–90. https://doi.org/10.1038/nature22901
Hume BCC, Voolstra CR, Arif C, D’Angelo C, Burt JA, Eyal G, Loya Y, Wiedenmann J (2016) Ancestral genetic diversity associated with the rapid spread of stress-tolerant coral symbionts in response to Holocene climate change. Proc Natl Acad Sci USA 113:4416–4421. https://doi.org/10.1073/pnas.1601910113
Hume BCC, Mejia-Restrepo A, Voolstra CR, Berumen ML (2020) Fine-scale delineation of Symbiodiniaceae genotypes on a previously bleached central Red Sea reef system demonstrates a prevalence of coral host-specific associations. Coral Reefs 39:583–601. https://doi.org/10.1007/s00338-020-01917-7
Iglesias-Prieto R, Trench RK (1994) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar Ecol Prog Ser 113:163–175
Iglesias-Prieto R, Matta JL, Robins WA, Trench RK (1992) Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc Natl Acad Sci USA 89:10302–10305. https://doi.org/10.1073/pnas.89.21.10302
IPCC (2023) Climate Change: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Core Writing Team, Lee H, Romero J (eds)). IPCC, Geneva, Switzerland, pp. 35–115. https://doi.org/10.59327/IPCC/AR6-978929169164
Jeong HJ, Lee SY, Kang NS, Yoo YD, Lim AS, Lee MJ, Kim HS, Yih W, Yamashita H, LaJeunesse TC (2014) Genetics and morphology characterize the dinoflagellate Symbiodinium voratum, n. sp., (Dinophyceae) as the sole representative of Symbiodinium clade E. J Eukaryot Microbiol 61:75–94. https://doi.org/10.1111/jeu.12088
Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature-induced bleaching or corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ 21:12129–21230. https://doi.org/10.1046/j.1365-3040.1998.00345.x
Kampmann H (2002) Photobiologische, energetische und genetische Aspekte des mutualistischen Zusammenlebens von Zooxanthellen (Symbiodinium sp.) und Steinkorallen im Golf von Aqaba, Jordanien. Ph.D. dissertation, University of Cologne, Cologne, Germany, 147 pp.
Karim W, Nakaema S, Hidaka M (2015) Temperature effects on the growth rates and photosynthetic activities of Symbiodinium cells. J Mar Sci Eng 3:368–381. https://doi.org/10.3390/jmse3020368
Karsten U, Herburger K, Holzinger A (2014) Desiccation, temperature and light tolerance in members of the aeroterrestrial green algal genus Interfilum (Streptophyta) from biogeographically different temperate soils. J Phycol 50:804–816. https://doi.org/10.1111/jpy.12210
Kirchhoff H (2014) Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos Trans R Soc Lond B Biol Sci 369:20130225. https://doi.org/10.1098/rstb.2013.0225
Klueter A, Trapani J, Archer FI, Mcllroy SE, Coffroth MA (2017) Comparative growth rates of cultured marine dinoflagellates in the genus Symbiodinium and the effects of temperature and light. PLoS ONE 12:e0187707. https://doi.org/10.1371/journal.pone.0187707
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28:2570-2580.e6. https://doi.org/10.1016/j.cub.2018.07.008
LaJeunesse TC, Wiedenmann J, Casado-Amezúa P, D’ambra I, Turnham KE, Nitschke MR, Oakley CA, Goffredo S, Spano CA, Cubillos VM, Davy SK (2022) Revival of Philozoon Geddes for host-specialized dinoflagellates, ‘zooxanthellae’, in animals from coastal temperate zones of northern and southern hemispheres. Eur J Phycol 57:166–180. https://doi.org/10.1080/09670262.2021.1914863
Lee MJ, Jeong HJ, Jang SH, Kang NS, Lee KH, Kim HS, Wham DC, LaJeunesse TC (2016) Most low-abundance “background” Symbiodinium spp. are transitory and have minimal functional significance for references symbiotic corals. Microb Ecol 71:771–783. https://doi.org/10.1007/s00248-015-0724-2
Lesser MP (1996) Elevated temperatures and ultraviolet radiation causes oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol Oceanogr 41:271–283. https://doi.org/10.4319/lo.1996.41.2.0271
Lesser MP (2011) Coral bleaching: causes and mechanisms. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Berlin, pp 405–419
Lichtenberg M, Larkum AWD, Kühl M (2016) Photosynthetic acclimation of Symbiodinium in hospite depends on vertical position in the tissue of the Scleractinian coral Montastrea curta. Front Microbiol 7:230. https://doi.org/10.3389/fmicb.2016.00230
Lindström K (1984) Effect of temperature, light and pH on growth, photosynthesis and respiration of the dinoflagellate Peridinium cinctum fa. westii in laboratory cultures. J Phycol 20:212–220. https://doi.org/10.1111/j.0022-3646.1984.00212.x
Maruyama S, Weis VM (2021) Limitations of using cultured algae to study Cnidarian-algal symbioses and suggestions for future studies. J Phycol 57:30–38. https://doi.org/10.1111/jpy.13102
Moberg F, Folke C (1999) Ecological goods and services of coral reef ecosystems. Ecol Econ 29:215–233. https://doi.org/10.1016/S0921-8009(99)00009-9
Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566. https://doi.org/10.1104/pp.125.4.1558
Nitschke MR, Craveiro SC, Brandão C, Fidalgo C, Serôdio J, Calado AJ, Frommlet JC (2020) Description of Freudenthalidium gen. nov. and Halluxium gen. nov. to formally recognize clades Fr3 and H as genera in the family Symbiodiniaceae (Dinophyceae). J Phycol 56:923–940. https://doi.org/10.1111/jpy.12999
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359. https://doi.org/10.1146/annurev.arplant.50.1.333
National Oceanic and Atmospheric Administration (NOAA). What is El Niño? Pacific Marine Environmental Laboratory. Available from: https://www.pmel.noaa.gov/elnino/. Accessed on 23 Nov, 2023.
Van Oppen MJH, Baker AC, Coffroth MA, Willis BL (2009) Bleaching resistance and the role of algal endosymbionts. In: van Oppen MJH, Lough JM (eds) Coral Bleaching. Ecol Stud 205:83–102. Springer, Berlin, Germany. https://doi.org/10.1007/978-3-540-69775-6_6
Parkinson JE, Baums IB (2014) The extended phenotypes of marine symbioses: ecological and evolutionary consequences of intraspecific genetic diversity in coral–algal associations. Front Microbiol 5:445. https://doi.org/10.3389/fmicb.2014.00445
Parkinson JE, Coffroth MA, LaJeunesse TC (2015) New species of Clade B Symbiodinium (Dinophyceae) from the greater Caribbean belong to different functional guilds: S. aenigmaticum sp. nov., S. antillogorgium sp. nov., S. endomadracis sp. nov., and S. pseudominutum sp. nov. J Phycol 51:850–858. https://doi.org/10.1111/jpy.12340
Pochon X, LaJeunesse TC (2021) Miliolidium n. gen, a new symbiodiniacean genus whose members associate with soritid foraminifera or are free‐living. J Eukaryot Microbiol 68: e12856. https://doi.org/10.1111/jeu.12856
Prelle LR, Schmidt I, Schimani K, Zimmermann J, Abarca N, Skibbe O, Juchem D, Karsten U (2022) Photosynthetic, respirational, and growth responses of six benthic diatoms from the Antarctic Peninsula as functions of salinity and temperature variations. Genes 13:1264. https://doi.org/10.3390/genes13071264
Quigley KM, Alvarez Roa C, Beltran VH, Leggat B, Willis BL (2021) Experimental evolution of the coral algal endosymbiont Cladocopium goreaui: lessons learnt across a decade of stress experiments to enhance coral heat tolerance. Restor Ecol 29:e13342. https://doi.org/10.1111/rec.13342
Rädecker N, Escrig S, Spangenberg JE, Voolstra CR, Meibom A (2023) Coupled carbon and nitrogen cycling regulates the cnidarian-algal symbiosis. Nat Commun 14:1–10. https://doi.org/10.1038/s41467-023-42579-7
Robinson JD, Warner ME (2006) Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrhophyta). J Phycol 42:586–579. https://doi.org/10.1111/j.1529-8817.2006.00232.x
Roth MS (2014) The engine of the reef: photobiology of the coral-algae symbiosis. Front Microbiol 5:422. https://doi.org/10.3389/fmicb.2014.00422
Rowan R, Powers DA (1991) A molecular genetic classification of zooxanthellae and the evolution of animal-algal symbioses. Science 251:1348–1351. https://doi.org/10.1126/science.251.4999.1348
Rurek M (2014) Plant mitochondria under a variety of temperature stress conditions. Mitochondrion 19:289–294. https://doi.org/10.1016/j.mito.2014.02.007
Russnak V, Rodriguez-Lanetty M, Karsten U (2021) Photophysiological tolerance and thermal plasticity of genetically different Symbiodniaceace endosymbiont species of Cnidaria. Front Mar Sci 8:657348. https://doi.org/10.3389/fmars.2021.657348
Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O (2008) Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc Natl Acad Sci USA 105:10444–10449. https://doi.org/10.1073/pnas.0708049105
Slot M, Rey-Sanchez C, Gerber S, Lichstein JW, Winter K, Kitajima K (2014) Thermal acclimation of leaf respiration of tropical trees and lianas: response to experimental canopy warming, and consequences for tropical forest carbon balance. Glob Change Biol 20:2915–2926. https://doi.org/10.1111/gcb.12563
Souter D, Planes S, Wicquart J, Logan M, Obura D, Staub F (2021) Status of coral reefs of the world: 2020 report. Global Coral Reef Monitoring Network (GCRMN) and International Coral Reef Initiative (ICRI). https://doi.org/10.59387/WOTJ9184
Suggett DJ, Goyen S, Evenhuis C, Szabó M, Pettay DT, Warner ME, Ralph PJ (2015) Functional diversity of photobiological traits within the genus Symbiodinium appears to be governed by the interaction of cell size with cladal designation. New Phytol 208:370–381. https://doi.org/10.1111/nph.13483
Suggett DJ, Warner ME, Leggat W (2017) Symbiotic dinoflagellate functional diversity mediates coral survival under ecological crisis. Trends Ecol Evol 32:735–745. https://doi.org/10.1016/j.tree.2017.07.013
Swain TD, Chandler J, Backman V, Marcelino L (2017) Consensus thermotolerance ranking for 110 Symbiodinium phylotypes: an exemplar utilization of a novel iterative partial-rank aggregation tool with broad application potential. Funct Ecol 31:172–183. https://doi.org/10.1111/1365-2435.12694
Takahashi S, Whitney S, Itoh S, Maruyama T, Badger M (2008) Heat stress causes inhibition of the de novo synthesis of antenna proteins and photobleaching in cultured Symbiodinium. Proc Natl Acad Sci USA 105:4203–4208. https://doi.org/10.1073/pnas.0708554105
Thornhill DJ, Kemp DW, Bruns BU, Fitt WK, Schmidt GW (2008) Correspondence between cold tolerance and temperate biogeography in a Western Atlantic Symbiodinium (Dinophyta) lineage 1. J Phycol 44:1126–1135. https://doi.org/10.1111/j.1529-8817.2008.00567.x
Van Oppen MJH, Lough JM (2018) Coral Bleaching. Patterns, Processes, Causes and Consequences. Springer International Publishing, New York, USA, p 356. https://doi.org/10.1007/978-3-319-75393-5
Voolstra CR, Suggett DJ, Peixoto RS, Parkinson JE, Quigley KM, Silveira CB, Sweet M, Muller EM, Barshis DJ, Bourne DG, Aranda M (2021) Extending the natural adaptive capacity of coral holobionts. Nat Rev Earth Environ 2:747–762. https://doi.org/10.1038/s43017-021-00214-3
Walsby AE (1997) Numerical integration of phytoplankton photosynthesis through time and depth in a water column. New Phytol 136:189–209. https://doi.org/10.1046/j.1469-8137.1997.00736.x
Warner ME, Fitt WK, Schmidt GW (1996) The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant Cell Environ 19:291–299. https://doi.org/10.1111/j.1365-3040.1996.tb00251.x
Acknowledgements
The authors thank Prof. Dr. Jörg C. Frommlet, University of Aveiro, Portugal, for providing endosymbiontic cultures, as well as Niklas Plag and Torben Bruhns, both University of Rostock, for technical support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
338_2024_2557_MOESM1_ESM.tif
Supplementary file1 Illustration of the custom-built photosynthesis-irradiance (PE) box used for all photosynthetic and respiratory measurements.(TIF 2555 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Röser, P., Glaser, K., Juchem, D. et al. Species-specific effects of light and temperature on photosynthesis and respiration among Symbiodiniaceae (Dinophyceae). Coral Reefs 43, 1523–1534 (2024). https://doi.org/10.1007/s00338-024-02557-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02557-x