Abstract
Increasingly frequent and severe bleaching events driven by climate change are decreasing coral populations worldwide. Recovery of these populations relies on reproduction by the survivors of such events including local and upstream larval sources. Yet, corals that survive bleaching may be impaired by sublethal effects that suppress reproduction, reducing larval input to reefs, and consequently impeding recovery. We investigated the impact of the 2020 mass-bleaching event on Acropora millepora reproduction on inshore, turbid reefs in Woppaburra sea Country (the Keppel Islands), to improve our understanding of the effects of bleaching on coral populations. A. millepora experienced high bleaching incidence but low mortality across the island group during this event and thus constituted an ideal population to investigate potential sublethal effects on reproductive output. Six months after the heat wave, and just prior to spawning, we collected, decalcified, and dissected samples from 94 tagged A. millepora colonies with a known 2020 bleaching response, to investigate the relationships between stress severity and reproduction. Despite having regained their pigmentation, we detected a significant reduction in fecundity in colonies that had bleached severely. Considering the impact of the bleaching event on the coral population sampled (i.e., mortality, bleaching severity and colony size), coupled with reductions in fecundity, we estimated a total decrease in population-level reproductive output of 21%. These results suggest that reduced reproductive output may impact recovery of coral populations following bleaching and should be considered alongside traditional estimates of coral mortality.
Similar content being viewed by others
Introduction
Coral reefs support key ecological functions and ecosystem services that are important for humans (Sheppard et al. 2005; Worm et al. 2006; Cinner et al. 2016). However, increasingly frequent climate-change driven disturbance events, including regional scale mass-coral bleaching, have negatively affected coral reefs globally and are challenging the ecosystem’s recovery potential (Hughes et al. 2017, 2018a; Sully et al. 2019). An estimated 50% of coral cover worldwide has been lost since the 1950s and a downward trajectory is predicted to continue throughout the twenty-first century (van Hooidonk et al. 2016; Eddy et al. 2021). Consequently, the capacity for coral reefs to support critical ecosystem services is declining (Hughes et al. 2018b, 2019; Eddy et al. 2021), and the future value of reef systems will depend on their persistence under future climate-change emission scenarios (Hoegh-Guldberg et al. 2007; Chen et al. 2015).
Recovery and persistence of reefs after disturbances is driven by the growth of surviving colonies (van Oppen et al. 2015) and by larval recruitment from sexual reproduction (Glynn et al. 2012). Larval supply comes from both mass-spawning events (i.e.,, the synchronous release of gametes by spawning corals at particular times of the year), and from brooding corals, which experience internal fertilization and then release fully developed larvae (Sakai et al. 2020). In both cases, reproduction is energetically costly, requiring a build-up of energy reserves prior to spawning (Leuzinger et al. 2003; Anthony et al. 2007). Coral eggs are comprised of proteins, carbohydrates, and lipids, which aid in embryo floatation and provide an energy source for the developing larvae (Harii et al. 2007, 2010). The accumulation of lipids and other macromolecules is directly impacted by the breakdown of the coral host-symbiont relationship during bleaching and the resulting decline in nutrition transfer from the symbiont to the coral host (Conlan et al. 2020; Rodrigues and Padilla-Gamiño 2022). Energy use by corals is also altered during bleaching and can lead to physiological trade-offs, including the differential partitioning of energy between gamete production and colony maintenance (Rodrigues and Padilla-Gamiño 2022). Due to the annual reproductive cycle in many coral species, oocyte maturation and/or spawning often coincides with summer heat-stress (Randall et al. 2020; Shikina and Chang 2016), increasing the potential for bleaching to impact reproductive output. Therefore, understanding how bleaching events influence the production of coral gametes, and subsequent supply of larvae to degraded reefs, can inform our understanding of the recovery capacity of coral populations and communities.
To date, three primary effects of heat-stress on coral reproduction have been documented and include, in decreasing order of severity: (i) complete failure of gametogenesis; (ii) a decrease in fecundity; and (iii) a decline in gamete viability. Firstly, complete failure to produce gametes is the most well-documented sublethal impact of bleaching on coral reproduction (Ward et al. 2002; Levitan et al. 2014) and has been recorded during heat-stress conditions even when colonies showed no visible signs of bleaching (Rodriguez-Troncoso et al. 2011). Secondly, a decline in metrics of reproductive output such as egg size and number has been observed in response to bleaching (Michalek-Wagner and Willis 2001; Baird and Marshall 2002; Ward et al. 2002; Cox 2007). For example, Jones and Berkelmans (2011) found that bleached Acropora millepora colonies produced smaller and 50% fewer eggs, and that the proportion of colonies within the population that were reproductive had declined. Thirdly, some studies have demonstrated an effect of bleaching on gamete and larval viability. For example, Hagedorn et al. (2016) documented a decline in sperm motility and fertilization rates following bleaching, while an increase in developmental abnormalities and a decline in larval survival in response to direct heat stress has been observed (Randall and Szmant 2009; Lenz et al. 2021). While most studies examine the immediate impacts of heat stress or bleaching on reproduction, how long these effects persist following heat stress and colony recovery remains unclear. Furthermore, variability in the persistence of these sublethal effects has been noted across species, geographic regions and environmental regimes. Therefore, continuing to investigate the potential long-term sub-lethal impacts of bleaching on species and reefs is critically important for predicting coral responses to future climate warming.
Severe and widespread bleaching occurred within Woppaburra sea Country (the Keppel Islands, southern inshore Great Barrier Reef (GBR)) in March—April 2020. Although mortality was low (Page et al. 2023), it was unknown whether the surviving corals suffered any impacts to reproduction in the spawning season following bleaching. Furthermore, individual bleaching responses were highly variable, and the relationship between bleaching severity and post-bleaching reproductive output is not well documented (Leinbach et al. 2021). To address these knowledge gaps, we tagged Acropora millepora colonies during bleaching and tracked their fates over six months. Colonies that spanned the full spectrum of bleaching phenotypes, from none to catastrophic, were then sampled, decalcified, and dissected to address the following research questions: (i) what is the relationship between bleaching severity and fecundity? (ii) how does post-bleaching fecundity compare with historical baselines? (iii) are bleaching severity, colony size, and coral mortality related?
Methods
Site selection, sample collection and decalcification
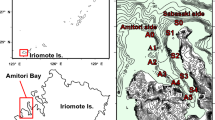
This study was conducted on fringing reefs in the Keppel Islands of the southern inshore GBR. Four sites were selected across the Island group: Great Keppel Island (GKI, 23.1030° S 150.5740° E), North Keppel Island (NKI, 23.0738° S, 150.8987° E), Halfway Island (HI, 23.1984° S, 150.9718° E), and Pumpkin Island (PI, 23.0927° S, 150.9011° E) (Fig. 1) and were at 1 to 5 m depth below lowest astronomical tide (LAT). Acropora millepora is a corymbose species common across Woppaburra sea Country where it has been extensively studied (Jones and Berkelmans 2011).
a Map of the Keppel Islands with four collection sites identified: North Keppel Island (NKI), Pumpkin Island (PI), Great Keppel Island (GKI), and Halfway Island (HI). Background color represents the mean temperature in February 2020 at the height of the heat wave, at 2.35 m depth, as modeled by eReefs (www.ereefs.aims.gov.au). Figure after Page et al. 2023. b Bleaching color scale, from 1 to 6, based on the CoralWatch Coral Health Chart (Siebeck et al. 2006), and corresponding representative colonies of each score, from in-water surveys in April 2020. Bleaching severity classifications from ‘none’ to ‘catastrophic’ were added alongside nominal scores from the CoralWatch chart, based on our results
Between 18 and 21 April 2020, at the height of the heatwave induced bleaching, 350 adult A. millepora colonies (~ 10—75 cm maximum diameter, average = 30 cm) were haphazardly tagged across sites, to capture a wide range of bleaching phenotypes within the population. Colonies were tagged at each of NKI (n = 100), PI (n = 101), HI (n = 99), and GKI (n = 50). During tagging, colonies were assigned an in situ ordinal bleaching score that ranged from 1 to 6, based on the CoralWatch Coral Health Chart (Siebeck et al. 2006) and were photographed (Fig. 1b).
Between 7 and 17 October 2020, approximately 1 month prior to the predicted coral spawning (realized on 9 Nov 2020 for colonies from the population (C. Randall personal observation)) and approximately 6 months following the height of bleaching, 311 tagged colonies were re-surveyed and the following data were recorded: (i) mortality status (dead or alive), (ii) percentage of live tissue remaining in intervals of 10% (i.e., 10—100% live tissue), (iii) maximum diameter (cm), (iv) maximum perpendicular diameter (cm), and (v) bleaching score (1–6), as described above. Colonies that were not sampled at the second time point (n = 39) were excluded from analysis. Estimated surface area of live tissue \(\left(SA\right)\) was calculated for each colony from the maximum diameter (\(a\)) and the maximum perpendicular diameter (\(b\)), both of which were recorded across a horizontal plane by a diver, using Eq. 1: \(SA = \pi \left( {\frac{1}{2}a} \right)\left( {\frac{1}{2}b} \right)\). Mean colony diameter was also calculated from \(a\) and \(b\).
During resurvey, three replicate branches were sampled from the central area of each colony to avoid the sterile zone found at the outer margins of Acropora colonies (Wallace 1985; Randall et al. 2021). Branches were collected using a small chisel or knife and were placed in pre-labeled sample bags with seawater. Immediately post dive, coral samples were transferred to a solution of 10% formaldehyde in 1 µm filtered seawater (FSW) (hereafter ‘formalin’). Samples were then transferred into 3% hydrochloric acid (HCl) in FSW solution for decalcification, and additional 3% HCl was added over a few days to replenish the weak acid until complete decalcification of the branches had occurred (decalcification durations varied from 3 to 10 days). Decalcified tissue samples were rinsed in FSW and stored in fresh formalin until dissection.
Sample dissection
Decalcified samples from a total of 94 colonies were dissected to assess fecundity (Fig. 2a). Colonies were systematically chosen for dissection to ensure an even representation across the range of bleaching phenotypes and, where possible, sites.
a Bleaching scores of disected colonies (n = 94) and b all tagged colonies (n = 350) recorded in April 2020 during the height of bleaching at four sites in the Keppel Islands. Colors of the bars represent bleaching scores from most severe (“catastrophic”; score = 1) to none (score = 6) (Siebeck et al. 2006). c Mean colony diameter against the April 2020 bleaching score with a locally estimated scatterplot smoothing (LOESS) curve and 95% confidence intervals in gray. d Logistic model predicting the probability of coral survival as a function of bleaching score, with 95% confidence intervals in gray. Note that points in (d) are jittered along the x-axis for readability
Branches were dissected under a Lecia M60 Stereomicroscope at 20 ×—40 × magnification. Measurements were taken at 25 × magnification from live image (5mp digital C-mount camera) within ToupView software. Maximum branch length was measured with digital calipers, and then a longitudinal section was cut through the middle of the branch, which was suspended in FSW in a wax dish, using a scalpel (Fig. 3a). The length of the sterile zone— the tip of Acropora branches where the newest growth lacks gonads (Wallace 1985)—was clearly visible from the longitudinal section and measured from the apical polyp tip to the nearest visibly fecund polyp (Fig. 3a). Ten polyps were haphazardly selected near the base of the branch, with the branch interior facing downward, in order to minimize bias in selecting polyps that had visible eggs; polyps were then removed with forceps and dissected as per Wallace 1985 (Fig. 3b). Very small polyps were avoided, although smaller than average polyps were sometimes selected as a result of the haphazard process and, in most instances, were observed to be reproductively mature. The number of polyps (out of 10) that had successfully produced oocytes was recorded. Then, the number of oocytes within each polyp was counted, and for each of the first three polyps containing eggs, the maximum diameter \(\left( d \right)\) was recorded for each egg observed. Egg volume (\(V\)) was then estimated using Eq. 2: \(V = \frac{4}{3}\pi \left( \frac{d}{2} \right)^{3}\), assuming a sphere.
Acropora millepora tissue dissections showing a a longitudinal cross section through a branch, and b a freshly isolated polyp (left) and a dissected polyp (right) showing spermatozoa (s) and oocytes (o) visible within both primary and secondary mesenteries. White scale bars in (a) and (b) represent 1 mm. Red line segment in (a) represents the length of the sterile zone. Blue line segment in (b) represents the maximum diameter of an oocyte within the mesentery. Photomicrographs: Christine Giuliano
Statistical analysis
All statistical analyses were completed in R (R Core Team 2022). Generalized linear mixed effects models (GLMM) utilizing a template model builder (Brooks et al. 2017) were used to model reproductive output. Models were created to test for the additive effects of bleaching score, site, and mean colony diameter on three reproductive metrics: (1) egg size, (2) number of eggs per polyp, and (3) number of eggs per fecund polyp. Replicate branch within colony, and colony within site were treated as nested random effects in the model of egg size. In the models of egg numbers, only colony within site was treated as a nested random effect due to a lack of convergence in the full model. All response variables (egg size, number of eggs per polyp, and number of eggs per fecund polyp) were modeled with a Gaussian distribution. A null model was formulated using only random effects and model selection was undertaken by comparing models with each combination of predictors against the null model, using second-order Akaike Information Criterion (AICc) in the MuMIn package (Bartoń, 2023). Analysis of Variance (ANOVA) was used to statistically compare models and validate model selection. Based on this model selection method, site and mean diameter were not included in the final models of number of eggs per polyp and number of eggs per fecund polyp. Model assumptions were assessed and validated using DHARMa residual analysis (Hartig 2021) and results were visualized using ‘ggplot2’ (Wickham 2016).
Colony size does not appear to determine reproductive output of each polyp in A. millepora once coral maturation is reached at approximately 15 cm in diameter (Hall and Hughes 1996; Baria et al. 2012). Therefore, two colonies from NKI that were less than 15 cm diameter were excluded from the analysis. Samples from HI were also removed from the site-specific models due to a comparatively small sample size (HI n = 4, PI n = 29, NKI n = 34, GKI n = 27). Therefore, a total of 88 colonies were included in the models testing for site-level variation, and 92 colonies were included in fecundity models.
To investigate the relationship between bleaching score and colony survival, a logistic regression with a binomial distribution and a logit link function was modeled using ‘glm’ from the ‘stats’ package and diagnostics were checked as described above (R Core Team 2022).
Historical data
Three years of historical A. millepora fecundity data from the Keppel Islands were used to establish a baseline of reproductive output prior to recent bleaching. Firstly, Tan et al. (2016) measured the number of eggs per polyp from haphazardly selected A. millepora colonies in a manner comparable to this study, in 2009 and 2010, prior to the 2016 and 2017 bleaching events. Secondly, following the sampling methods described above, a single branch from 49 haphazardly sampled colonies from 10 sites across the Keppel Islands in October 2019 (6 months before bleaching) were dissected, 12–19 days prior to 2019 spawning. To determine whether reproductive output in 2020 differed from these baselines, we modeled the number of eggs per polyp against year using a general linear model with a Gaussian distribution, as described above.
Estimate of population level reduction in fecundity
Based on the results of this study, a population level fecundity reduction was estimated from the bleaching data that were collected from all 310 colonies surveyed during bleaching in April 2020. Firstly, a hypothetical population-level fecundity potential was calculated, in the absence of bleaching. To do this, we first assumed a sterile zone of length 7.3 mm around the colony perimeter as per the mean sterile zone measured from all replicates. We then re-calculated the maximum fecund diameter (\(a\)) and maximum fecund perpendicular diameter \(\left( b \right)\) for each colony by subtracting 14.6 mm (7.3 mm on each side) from each metric and used those values to calculate a ‘fecund SA’ in cm2 for each colony using Eq. 1. The total number of fecund polyps per colony was then estimated by multiplying the fecund SA (of planar area measured by diameter (in cm2)) by the average density of polyps in Acropora millepora (87 polyps cm−2; Hall and Hughes 1996). From this, the modeled number of eggs per polyp, assuming no bleaching (score = 6; 7.47 eggs per polyp), was multiplied by the number of reproductive polyps per colony, to create a baseline assumption of a colony’s potential reproductive output, if healthy. Then, to account for bleaching and partial mortality, the number of fecund polyps for each colony was reduced by the % reduction in egg number estimated for that colony’s bleaching score and then reduced by the % partial mortality observed, to estimate a realized reproductive output following bleaching. For colonies that suffered complete mortality, realized reproductive output was zero. Finally, the percentage reduction between the hypothetical reproductive output and the estimated realized reproductive output for all colonies combined was calculated, providing an estimate of the impact of the 2020 bleaching event on population-level fecundity. We note that this method assumes a planar SA and thus likely underestimates the colony-level reproductive potential, although the estimated percentage reduction should scale proportionally.
Comparison of diver assessed and photo-surveyed bleaching scores
To assess whether field images of colonies could be used to accurately identify bleaching severity, bleaching scores were estimated from images taken in April 2020 using Coral Point Count with Excel Extensions (CPCe), from 10 randomly placed points overlaid on each colony, excluding those points that fell on the growing tips of the colonies, which are naturally paler than the surrounding colony (Kohler and Gill 2006). The relationship between in situ diver assessed and ex situ photo-surveyed bleaching scores was tested using a Pearson’s correlation coefficient with the function cor.test in base R (R Core Team 2022).
Results
Of the colonies phenotyped during the height of bleaching (April 2020), 61% had a severe bleaching response (category = 2, n = 214) while 15% had a catastrophic response (category = 1, n = 53), together accounting for 76% of all colonies scored (Fig. 2b). Only 4.5% of colonies did not visually bleach (category = 6, n = 16). Whole-colony mortality was highest at PI (17%, n = 16) while no whole-colony mortality was observed at NKI (SI Table 1, Page et al. 2023). The likelihood of survival significantly increased as a function of bleaching score (GLM: z = 3.437, p < 0.001) (Fig. 2d); only ‘catastrophically’ and ‘severely’ bleached colonies (scores of 1 and 2, respectively) suffered whole-colony mortality (2.9% in score 1 (n = 9) and 3.5% in score 2 (n = 11), which equated to an overall mortality of 6.5% (n = 20). Partial mortality ranged from 10 to 90%, but occurred rarely (incidence of 2%), and only in severely bleached colonies (score of 2, n = 6). As colony size increased, the bleaching response became more severe (Fig. 2c). Nearly all colonies had recovered by October, with an average score of 5 (‘negligible’ bleaching) at all sites at that time, with only two colonies retaining a ‘mild’ level of bleaching (score 3, n = 2).
Egg number
Polyp fecundity (number of eggs per polyp and number of eggs per fecund polyp) significantly differed by bleaching score, but not by site or colony size (Table 1, Fig. 4c, d). Egg output per polyp decreased by approximately 21% from the least bleached (7.5 eggs per polyp) to the most bleached (5.9 eggs per polyp) colonies (SE = 0.77, p = < 0.05), with similar results in the egg output per fecund polyp model (SE = 0.63, p = < 0.05) (Table 1). While egg output differed in the overall models between scores of 1 and 6, pairwise post-hoc Tukey tests showed no significant pairwise differences in either model.
Modeled egg size (a, b) and number of eggs per polyp (c, d) by bleaching field score (a, c) and site (b, d) in Acropora millepora. Asterisk indicates a bleaching score that was significantly different from score 6 in the model (p < 0.05). (e) Fecundity in the Keppel Island’s A. millepora population before (2009, 2010; Tan et al. 2016, 2019 Giuliano, unpublished) and after (2020; present study) bleaching
Egg size
Bleaching score was not a significant predictor of egg size, although site and colony diameter were (Fig. 4a, b). Eggs from GKI colonies were 0.05 mm larger than eggs from NKI colonies (SE = 0.01, p < 0.0001) and 0.04 mm larger than eggs from PI colonies (SE = 0.01, p < 0.01) (Table 1). These size estimates equate to an approximate 10% difference in egg diameter, and a consequent 25% difference in egg volume between GKI and NKI.
Population-level fecundity
Based on the estimate of fecundity for each bleaching score, combined with whole-colony and partial mortality, we estimated a 21% reduction in total oocyte output of the population six months after the 2020 bleaching event.
Coral fecundity past and present
Mean number of eggs per fecund polyp in 2020 did not differ from pre-bleaching baselines (Fig. 4e). Samples from 2019 and unbleached samples in 2020 (scores of 5 and 6) had a higher mean polyp fecundity than the historic baseline, but bleached colonies in 2020 (scores of 1 and 2) had a similar or lower polyp fecundity than historic baselines (compare Fig. 4c and 4e).
Comparison of diver assessed and photo-surveyed bleaching scores
Diver assessed and photo surveyed (CPCe) bleaching scores were tightly and positively correlated (t = 29.99, df = 348, p < 0.001, r = 0.85) (Fig. S1). The greatest variance in CPCe score was found for colonies with field scores of 2 and 3, while the least variance was seen for colonies at either end of the scale (scores 1 and 6). However, some colonies scored as ‘severe’ (score = 2) by divers in situ were scored as not bleached by the CPCe method, indicating the potential for some underestimation of bleaching from images (Fig. S1).
Discussion
Severe bleaching occurred throughout Woppaburra sea Country (the Keppel Islands) during the 2020 marine heatwave, but mortality of Acropora millepora was low (~ 6.5% this study; Page et al. 2023). Despite recovery by the time of coral spawning six months later, we found that heavily bleached colonies experienced a significant reduction in reproductive output in the form of depressed egg numbers, while egg size was conserved, resulting in an estimated 21% reduction in population-level fecundity. These sublethal effects often go undetected and highlight the importance of tracking recovery metrics beyond survival when assessing the impacts of bleaching on coral populations.
Bleaching and reproductive output
Generally, the capacity for gamete production was maintained in the A. millepora population following bleaching, with only one survivor suffering complete reproductive failure. Early research into the effects of bleaching on reproduction reported mass reproductive cessation, among other effects (Baird and Marshall 2002; Ward et al. 2002). Yet since this early research, fecundity responses have varied greatly depending on the region, the species, and the extent and timing of bleaching (i.e. Cox 2007; Jones and Berkelmans 2011; Levitan et al. 2014), and susceptibility has likely changed due to local adaptation. Interestingly, while not reported in Tan et al. 2016, partial reproductive failure (i.e., some non-reproductive polyps within reproductive branches, or some non-reproductive branches within a reproductive colony) was observed extensively here. This response may be the result of differential allocation of limited resources to certain polyps or branches (Leinbach et al. 2021). However, Tan et al. 2016 preferentially selected polyps containing visible eggs, potentially underestimating partial reproductive failure. While an increase in reproductive output in the years after bleaching has also been reported (Armoza-Zvuloni et al. 2011), our study did not indicate a last-ditch effort to reproduce as evidenced by the similarity in overall reproductive output among years (Fig. 4e).
Egg size appeared largely unaffected by bleaching (Fig. 4a), but both the mean number of eggs per polyp and the mean number of eggs per fecund polyp significantly decreased when colonies that were not bleached (score 6) were compared to those that catastrophically bleached (score 1), equating to a 21% decrease in total egg production (Fig. 4c; Table 1). Therefore, colonies appeared to have preferentially conserved egg size over egg number. Egg size is often used in corals as an indicator of health, as smaller eggs are provisioned with less lipids, are less likely to fertilize, and can have shorter larval durations (Levitan 2006). The conservation of egg size, but a decrease in egg quantity, is consistent with another study on A. millepora (Jones and Berkelmans 2011), and similar findings for another Acropora species have led researchers to conclude that this size-number trade-off may be an Acropora-specific trait (Leinbach et al. 2021). The population at large reflected this response; however, variation in egg size among sites (Table 1) suggests that environmental factors may also influence the degree to which corals are able to provision their eggs. For example, eggs from GKI were nearly 25% larger in volume than eggs from NKI (Fig. 4b), despite similar levels of bleaching. Interestingly, NKI had some of the highest cumulative heat-stress during the 2020 bleaching event (Page et al. 2023), suggesting that this environmental stress may have influenced the provisioning of eggs, irrespective of bleaching score.
The concept of coral species as winners and losers in response to heat stress is not new to coral reef ecology (Loya et al. 2001; van Woesik et al. 2011), but the Keppel Islands population of A. millepora appears to act as a ‘winner’ despite exhibiting morphological, biogeographic, and physiological characteristics of a typical ‘loser’: the corymbose morphology of A. millepora is complex in nature, the Keppel Islands lie inshore and are less than 20 km from inputs of terrestrial sediments, nutrients and pollutants, and the species that dominate reefs in this region are considered sensitive, weedy, and fast-growing (Jones and Berkelmans 2014; Thompson et al. 2022; Page et al. 2023). Despite these factors, A. millepora remains resilient in this region. Previous studies have shown that an increase in heterotrophically-derived nutrition can prevent mortality and aid in rebuilding energy reserves up to a year after bleaching, which is necessary for provisioning eggs (Grottoli et al. 2006; Hughes and Grottoli 2013). Anthony (1999) demonstrated that A. millepora can have high heterotrophic plasticity; therefore, the highly turbid nature of these reefs may provide greater opportunities for heterotrophic feeding than on lower turbidity mid-shelf and offshore reefs.
Bleaching and coral mortality
Despite the severe bleaching exhibited by nearly 75% of A. millepora colonies, there was some variability in bleaching response within the population (Fig. 2). In general, mortality remained low, and recovery to normal coloration (scores of 5 and 6) occurred for nearly all colonies within six months. Differential bleaching and survival through a marine heat wave can be driven by many factors, including variability in the prevalence of heat-tolerant symbiont communities (Ziegler et al. 2018; Rowan 2004; Jones et al. 2008). For example, the dominant genera of Symbiodiniaceae hosted can significantly affect coral heat tolerance (Pelosi et al. 2021), and symbiont shuffling following bleaching has aided in the speed of coral recovery following past bleaching in the Keppel Islands (Jones et al. 2008). Thus, the low mortality observed in the Keppel Islands in 2020 may be due to plasticity in symbiont communities hosted (Jones et al. 2008; Sweet 2014; Bay et al. 2016). The combination of Cladocopium C3 with Durisdinium-dominated colonies may have also provided some level of heat resilience to A. millepora colonies in this region, as has been previously identified (Berkelmans and van Oppen 2006; Jones et al. 2008; Bay et al. 2016). Future studies could use amplicon sequencing of multiple markers (Nitschke et al. 2022) or map reads from whole genome sequencing studies in samples collected over time to better understand the role of Symbiodiniaceae in driving bleaching resilience and recovery. Host-specific processes, such as host-environmental memory (Hackerott et al. 2021), phenotypic plasticity (Bellantuono et al. 2012) and genetic adaptation to bleaching recovery (van Oppen and Blackall 2019, Marhoefer et al. 2021) may have also contributed to rapid recovery and high survival in this population.
An alternative explanation for low mortality following severe bleaching discussed in Page et al. (2023) is that high turbidity in the region, coupled with large tidal ranges, may have facilitated recovery via three mechanisms: (1) turbidity could have reduced bleaching severity by reducing irradiance stress during periods of anomalously high seawater temperatures (Cacciapaglia and van Woesik 2016); (2) increased heterotrophic feeding through the deposition of particulate organic material could have aided recovery (Grottoli et al. 2006); and (3) higher levels of mass transfer with tidal flow may have helped alleviate the build-up of superoxide radicals during bleaching (Loya et al. 2001). Shading, feeding and increased flow can all contribute to the resilience of inshore corals, which in this region are known for their exceptional growth rates (Diaz-Pulido et al. 2009). Finally, the highly disturbed nature of the system may have already resulted in local adaptation. Reefs in the Keppel Islands are subjected to frequent and varied stresses (Diaz-Pulido et al. 2009; Thompson et al. 2022; Page et al. 2023). Therefore, it is likely that some level of local adaptation to thermal stress has already occurred, and colonies of A. millepora in the Keppel Islands have been shown to suffer lower background mortality than in other areas of the GBR (Tan et al. 2018). The 1998 bleaching event caused nearly 32% whole-colony mortality of fate-tracked A. millepora colonies on the GBR (Baird and Marshall 2002). Twenty-two years later, with more heat accumulation than in 1998, mortality was fivefold lower in the Keppel Islands, suggestive of their higher tolerance to heat stress than central-sector populations of A. millepora historically. Finally, below-average temperatures in the winter months following bleaching also likely offered a reprieve from stress (Randall and van Woesik 2015; Page et al. 2023), allowing the symbiotic relationship between the coral and their symbionts to recover quickly.
Comparison of diver assessed and photo-surveyed bleaching scores
There was a strong correlation between the diver-assessed and photo-surveyed scoring methods indicating that the photo-based bleaching assessment captured the scale and severity of bleaching well (Fig. S1). However, occasional discrepancies in diver-assessed scores compared with photo-surveyed scores suggests that the photo-survey method may miss some bleaching, particularly in highly turbid and low-light conditions. For example, one colony scored by divers as ‘severely’ bleached was categorized as ‘negligible’ by the photo-survey method (Fig. S1). Applying uniform lighting near the base of the colonies may significantly improve visibility of interior tissue for ex situ assessments, increasing accuracy. Thus, despite the occasional mismatch, the photo-survey method was fairly reliable and suggests that this approach may prove useful when assessing large datasets or studying large-scale bleaching patterns, particularly with minor improvements in the method e.g., standard camera settings and white balancing using a white/black/gray scale (Hoogenboom et al. 2017). The photo-assessment technique can be easily taught and incorporated into citizen science efforts to increase community involvement in reef monitoring and broaden the scale of rapid assessments required during heat waves, while simultaneously increasing community awareness of the current state of reefs in their region.
Conclusion
Understanding the impacts of bleaching on coral fitness is important for improving predictions of coral population and reef trajectories under climate change. Yet quantifying the impacts on longevity, growth and reproductive output are challenging and are often not captured in acute heat-response studies. Our results suggest that the reproductive output of the Keppel Islands A. millepora population was reduced by 21% in the year following the 2020 mass-bleaching event. Surviving corals are vulnerable to the often under-reported and overlooked sublethal impacts that can persist for a significant period (Johnston et al. 2020), and these effects may be further amplified in populations not adapted to such a highly disturbed and heterotrophic system. Corals that survive the increasing frequency and severity of heat waves predicted under climate change will likely have reduced fitness and reproductive output (Baird and Marshall 2002, Hagedorn et al. 2016, Johnston et al. 2020, Leinbach et al. 2021). Thus, further studies that assess the impacts of bleaching on coral fitness will improve predictions of coral populations and reef trajectories into the future.
Data availability
Data are available at the following public archive: https://apps.aims.gov.au/metadata/view/3bbb9779-2179-499d-b27c-77f934e8915d
References
Anthony KRN (1999) Coral suspension feeding on fine particulate matter. J Exp Mar Biol Ecol 232:85–106
Anthony KRN, Connolly SR, Hoegh-Guldberg O (2007) Bleaching, energetics, and coral mortality risk: effects of temperature, light, and sediment regime. Limnol Oceanogr 52:716–726
Armoza-Zvuloni R, Segal R, Kramarsky-Winter E, Loya Y (2011) Repeated bleaching events may result in high tolerance and notable gametogenesis in stony corals: Oculina patagonica as a model. Mar Ecol Prog Ser 426:149–159
Baird A, Marshall P (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Baria MV, Dela Cruz D, Villanueva R, Guest J (2012) Spawning of three-year-old Acropora millepora corals reared from larvae in Northwestern Philippines. Bull Mar Sci 88:6162
Bartoń K (2023) MuMIn: Multi-model inference. R package version 1.47.5
Bay LK, Doyle J, Logan M, Berkelmans R (2016) Recovery from bleaching is mediated by threshold densities of background thermo-tolerant symbiont types in a reef-building coral. Royal Soc Open Sci 3:160322
Bellantuono AJ, Hoegh-Guldberg O, Rodriguez-Lanetty M (2012) Resistance to thermal stress in corals without changes in symbiont composition. Proc Royal Soc b: Biol Sci 279:1100–1107
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc Royal Soc b: Biol Sci 273:2305–2312
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) GlmmTMB balances speed and lexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Cacciapaglia C, van Woesik R (2016) Climate-change refugia: shading reef corals by turbidity. Glob Change Biol 22:1145–1154
Chen PY, Chen CC, Chu L, McCarl B (2015) Evaluating the economic damage of climate change on global coral reefs. Glob Environ Change 30:12–20
Cinner J, Huchery C, MacNeil M et al (2016) Bright spots among the world’s coral reefs. Nature 535:416–419
Conlan JA, Bay LK, Jones A, Thompson A, Francis DS (2020) Seasonal variation in the lipid profile of Acropora millepora at Halfway Island, Great Barrier Reef. Coral Reefs 39:1753–1765
Cox EF (2007) Continuation of sexual reproduction in Montipora capitata following bleaching. Coral Reefs 26:721–724
Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, Kline DI, Weeks S, Evans RD, Williamson DH, Hoegh-Guldberg O (2009) Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4:e5239
Eddy TD, Lam VWY, Reygondeau G, Cisneros-Montemayor AM, Greer K, Palomares MLD, Bruno JF, Ota Y, Cheung WWL (2021) Global decline in capacity of coral reefs to provide ecosystem services. One Earth 4:1278–1285
Glynn PW, Colley SB, Maté JL, Baums IB, Feingold JS, Cortés J, Guzmán HM, Afflerbach JC, Brandtneris VW, Ault JS (2012) Reef coral reproduction in the equatorial eastern Pacific: Costa Rica, Panamá, and the Galápagos Islands (Ecuador). VII. Siderastreidae, Psammocora stellata and Psammocora profundacella. Mar Biol 159:1917–1932
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189
Hackerott S, Martell HA, Eirin-Lopez JM (2021) Coral environmental memory: causes, mechanisms, and consequences for future reefs. Trends Ecol Evol 36:1011–1023
Hagedorn M, Carter V, Lager C, Camperio J, Dygert A, Schleiger R, Henley E (2016) Potential bleaching effects on coral reproduction. Reprod Fertil Dev 28:1061
Hall VR, Hughes TP (1996) Reproductive strategies of modular organisms: comparative studies of reef-building corals. Ecology 77:950–963
Harii S, Nadaoka K, Yamamoto M, Iwao K (2007) Temporal changes in settlement, lipid content and lipid composition of larvae of the spawning hermatypic coral Acropora tenuis. Mar Ecol Prog Ser 346:89–96
Harii S, Yamamoto M, Hoegh-Guldberg O (2010) The relative contribution of dinoflagellate photosynthesis and stored lipids to the survivorship of symbiotic larvae of the reef-building corals. Mar Biol 157:1215–1224
Hartig F., (2021). DHARMa: Residual diagnostics for hierarchical (Multi-Level /Mixed) regression models. R package version 0.4.3. https://cran.r-project.org/package=DHARMa
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hoogenboom MO, Frank GE, Chase TJ, Jurriaans S, Álvarez-Noriega M, Peterson K, Critchell K, Berry KLE, Nicolet KJ, Ramsby B, Paley AS (2017) Environmental drivers of variation in bleaching severity of Acropora species during an extreme thermal anomaly. Front Mar Sci 4:376
Hughes AD, Grottoli AG (2013) Heterotrophic Compensation: A possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress? PLoS ONE 8:e81172
Hughes TP, Kerry JT, Álvarez-Noriega M et al (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G (2018a) Global warming transforms coral reef assemblages. Nature 556:492–496
Hughes TP, Kerry JT, Simpson T (2018b) Large-scale bleaching of corals on the Great Barrier Reef. Ecology 99:501
Hughes TP, Kerry JT, Baird AH, Connolly SR, Chase TJ, Dietzel A, Hill T, Hoey AS, Hoogenboom MO, Jacobson M, Kerswell A, Madin JS, Mieog A, Paley AS, Pratchett MS, Torda G, Woods RM (2019) Global warming impairs stock–recruitment dynamics of corals. Nature 568:387–390
Johnston EC, Counsell CWW, Sale TL, Burgess SC, Toonen RJ (2020) The legacy of stress: coral bleaching impacts reproduction years later. Funct Ecol 34:2315–2325
Jones AM, Berkelmans R (2011) Tradeoffs to thermal acclimation: energetics and reproduction of a reef coral with heat tolerant Symbiodinium type-D. J Mar Biol 2011:e185890
Jones AM, Berkelmans R (2014) Flood impacts in Keppel Bay, Southern Great Barrier Reef in the aftermath of cyclonic rainfall. PLoS ONE 9:e84739
Jones AM, Berkelmans R, van Oppen MJH, Mieog JC, Sinclair W (2008) A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc Royal Soc b: Biol Sci 275:1359–1365
Kohler KE, Gill SM (2006) Coral point count with excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269
Leinbach SE, Speare KE, Rossin AM, Holstein DM, Strader ME (2021) Energetic and reproductive costs of coral recovery in divergent bleaching responses. Sci Rep 11:23546
Lenz EA, Bartlett LA, Stathakopoulos A, Kuffner IB (2021) Physiological differences in bleaching response of the coral Porites astreoides along the Florida Keys Reef tract during high-temperature stress. Front Mar Sci 8:1–14
Leuzinger S, Anthony KRN, Willis BL (2003) Reproductive energy investment in corals: scaling with module size. Oecologia 136:524–531
Levitan DR (2006) The relationship between egg size and fertilization success in broadcast-spawning marine invertebrates. Integr Comp Biol 46:298–311
Levitan D, Boudreau W, Jara J, Knowlton N (2014) Long-term reduced spawning in Orbicella coral species due to temperature stress. Mar Ecol Prog Ser 515:1–10
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Marhoefer SR, Zenger KR, Strugnell JM, Logan M, van Oppen MJH, Kenkel CD, Bay LK (2021) Signatures of adaptation and acclimatization to reef flat and slope habitats in the coral Pocillopora damicornis. Front Mar Sci 8:1–12
Michalek-Wagner K, Willis BL (2001) Impacts of bleaching on the soft coral Lobophytum compactum. I. Fecundity, fertilization and offspring viability. Coral Reefs 19:231–239
Nitschke MR, Rosset SL, Oakley CA, Gardner SG, Camp EF, Suggett DJ, Davy SK (2022) The diversity and ecology of Symbiodiniaceae: a traits-based review. Adv Mar Biol 92:55–127
Page CA, Giuliano C, Bay LK, Randall CJ (2023) High survival following bleaching underscores the resilience of a frequently disturbed region of the Great Barrier Reef. Ecosphere 14:e4280
Pelosi J, Eaton KM, Mychajliw S, terHorst CP, Coffroth MA (2021) Thermally tolerant symbionts may explain Caribbean octocoral resilience to heat stress. Coral Reefs 40:1113–1125
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Randall C, Szmant A (2009) Elevated temperature affects development, survivorship, and settlement of the Elkhorn coral, Acropora palmata (Lamarck 1816). Biol Bull 217:269–282
Randall CJ, van Woesik R (2015) Contemporary white-band disease in Caribbean corals driven by climate change. Nat Clim Chang 5:375–379
Randall CJ, Negri AP, Quigley KM, Foster T, Ricardo GF, Webster NS, Bay LK, Harrison PL, Babcock RC, Heyward AJ (2020) Sexual production of corals for reef restoration in the Anthropocene. Mar Ecol Prog Ser 635:203–232
Randall CJ, Giuliano C, Page CA (2021) No bundles beyond this point: the coral sterile zone. Bull Mar Sci 97:75–76
Rodrigues LJ, Padilla-Gamiño JL (2022) Trophic provisioning and parental trade-offs lead to successful reproductive performance in corals after a bleaching event. Sci Rep 12:18702
Rodríguez-Troncoso AP, Carpizo-Ituarte E, Leyte-Morales GE, Chi-Barragán G, Tapia-Vázquez O (2011) Sexual reproduction of three coral species from the Mexican South Pacific. Mar Biol 158:2673–2683
Rowan R (2004) Thermal adaptation in reef coral symbionts. Nature 430:742–742
Sakai Y, Hatta M, Furukawa S, Kawata M, Ueno N, Maruyama S (2020) Environmental factors explain spawning day deviation from full moon in the scleractinian coral Acropora. Biol Let 16:20190760
Sheppard C, Dixon DJ, Gourlay M, Sheppard A, Payet R (2005) Coral mortality increases wave energy reaching shores protected by reef flats: examples from the Seychelles. Estuar Coast Shelf Sci 64:223–234
Shikina S, Chang CF (2016) Sexual Reproduction in Stony Corals and Insight into the Evolution of Oogenesis in Cnidaria. In: Goffredo S, Dubinsky Z (eds) The Cnidaria, Past, Present and Future: The world of Medusa and her sisters. Springer International Publishing, Cham, pp 249–268
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a color reference card. Coral Reefs 25:453–460
Sully S, Burkepile DE, Donovan MK, Hodgson G, van Woesik R (2019) A global analysis of coral bleaching over the past two decades. Nat Commun 10:1–5
Sweet MJ (2014) Symbiodinium diversity within Acropora muricata and the surrounding environment. Mar Ecol 35:343–353
Tan C, Pratchett M, Bay L, Graham E, Baird A (2016) Spatial and temporal variation in fecundity among populations of Acropora millepora on the Great Barrier Reef. Mar Ecol Prog Ser 561:147–153
Tan CH, Pratchett MS, Bay LK, Graham EM, Baird AH (2018) Biennium horribile: very high mortality in the reef coral Acropora millepora on the Great Barrier Reef in 2009 and 2010. Mar Ecol Prog Ser 604:133–142
Thompson A, Davidson J, Logan M, Coleman G (2022) Marine monitoring program: annual report inshore coral reef monitoring 2020–21.
van Oppen MJH, Blackall LL (2019) Coral microbiome dynamics, functions and design in a changing world. Nat Rev Microbiol 17:557–567
van Hooidonk R, Maynard J, Tamelander J, Gove J, Ahmadia G, Raymundo L, Williams G, Heron SF, Planes S (2016) Local-scale projections of coral reef futures and implications of the Paris agreement. Sci Rep 6:39666
van Oppen MJH, Lukoschek V, Berkelmans R, Peplow LM, Jones AM (2015) A population genetic assessment of coral recovery on highly disturbed reefs of the Keppel Island archipelago in the southern Great Barrier Reef. PeerJ 3:e1092
van Woesik R, Sakai K, Ganase A, Loya Y (2011) Revisiting the winners and the losers a decade after coral bleaching. Mar Ecol Prog Ser 434:67–76
Wallace CC (1985) Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus Acropora. Mar Biol 817:217–233
Ward S, Harrison P, and Hoegh-Guldberg O (2002) Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress.9th International Coral Reef Symposium, Bali, Indonesia, 23–27
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JBC, Lotze HK, Micheli F, Palumbi SR, Sala E, Selkoe KA, Stachowicz JJ, Watson R (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314:787–790
Ziegler M, Eguíluz VM, Duarte CM, Voolstra CR (2018) Rare symbionts may contribute to the resilience of coral–algal assemblages. ISME J 12:161–172
Acknowledgements
We acknowledge the Woppaburra People as the traditional Custodians of the Keppel Islands where this research took place. We pay our respects to their Elders past, present, and emerging and acknowledge their continuing spiritual connection to sea Country. All research was conducted with free prior and informed consent (FPIC) from the Woppaburra Traditional Use of Marine Resources Association (TUMRA) committee and was permitted under the Great Barrier Reef Marine Park Authority (GBRMPA) permit G19/43148.1. We thank the crew of the R.V. Cape Ferguson and staff of the Konomie Island Environmental Education Center for field support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was funded by the BHP—AIMS Australian Coral Reef Resilience Initiative.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Briggs, N.D., Page, C.A., Giuliano, C. et al. Dissecting coral recovery: bleaching reduces reproductive output in Acropora millepora. Coral Reefs (2024). https://doi.org/10.1007/s00338-024-02483-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00338-024-02483-y