Abstract
Dravet syndrome is a developmental and epileptic encephalopathy (DEE) characterized by intractable seizures, comorbidities related to developmental, cognitive, and motor delays, and a high mortality burden due to sudden unexpected death in epilepsy (SUDEP). Most Dravet syndrome cases are attributed to SCN1A haploinsufficiency, with genetic modifiers and environmental factors influencing disease severity. Mouse models with heterozygous deletion of Scn1a recapitulate key features of Dravet syndrome, including seizures and premature mortality; however, severity varies depending on genetic background. Here, we refined two Dravet survival modifier (Dsm) loci, Dsm2 on chromosome 7 and Dsm3 on chromosome 8, using interval-specific congenic (ISC) mapping. Dsm2 was complex and encompassed at least two separate loci, while Dsm3 was refined to a single locus. Candidate modifier genes within these refined loci were prioritized based on brain expression, strain-dependent differences, and biological relevance to seizures or epilepsy. High priority candidate genes for Dsm2 include Nav2, Ptpn5, Ldha, Dbx1, Prmt3 and Slc6a5, while Dsm3 has a single high priority candidate, Psd3. This study underscores the complex genetic architecture underlying Dravet syndrome and provides insights into potential modifier genes that could influence disease severity and serve as novel therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dravet syndrome is a developmental and epileptic encephalopathy (DEE) that arises during infancy (Dravet 2011). Initial seizures often occur in the context of a fever; however, the syndrome evolves to include afebrile seizure types. Seizures are often prolonged and respond poorly to conventional anticonvulsants (Dravet and Oguni 2013). Beyond seizures, individuals with Dravet syndrome exhibit several comorbidities, including developmental delay, intellectual disability, motor impairment, and behavioral and/or psychiatric issues, each impacting quality of life (Darra et al. 2019; Feng et al. 2024; Villas et al. 2017). Dravet syndrome is associated with a significantly elevated mortality risk, ranging from 4.4–17.5%, typically occurring during childhood. These premature deaths primarily result from sudden unexplained death in epilepsy (SUDEP) (15–53% of cases) or status epilepticus (SE) (11.5–36% of cases) (Cooper et al. 2016; Dravet et al. 2005; Sakauchi et al. 2011a, b; Strzelczyk et al. 2023).
Over 80% of individuals with Dravet syndrome have de novo pathogenic variants in the SCN1A gene that encodes the NaV1.1 voltage-gated sodium channel (Depienne et al. 2009; Zuberi et al. 2011). SCN1A haploinsufficiency is the major mechanism underlying Dravet syndrome (Gallagher et al. 2024). Despite a shared genetic basis, there is a range of clinical severity among individuals with SCN1A haploinsufficiency (Depienne et al. 2010, 2009; Goldberg-Stern et al. 2014; Guerrini et al. 2010; Nabbout et al. 2003; Osaka et al. 2007; Yordanova et al. 2011; Yu et al. 2010). This suggests that clinical phenotypes are influenced by other factors, including genetic modifiers and environmental factors (de Lange et al. 2020; Hammer et al. 2017; Kearney 2011).
Dravet syndrome can be modeled in mice by heterozygous deletion of Scn1a. Several mouse models with varying deletions have been generated and share similar phenotypes, including spontaneous and hyperthermia-induced seizures (Miller et al. 2014; Ogiwara et al. 2007; Yu et al. 2006). Similarly, heterozygous knock-in of loss-of-function missense variants can also recapitulate core Dravet syndrome features (Ricobaraza et al. 2019). Both knockout and knock-in models have a high risk of sudden unexpected death following a seizure in otherwise healthy animals (SUDEP-like) (Kalume et al. 2013; Miller et al. 2014; Ricobaraza et al. 2019; Yu et al. 2006). In the knockout models, the highest incidence of SUDEP-like deaths occurs early in life, with approximately 50% mortality by one month of age (Kalume et al. 2013; Miller et al. 2014).
A consistent feature among Dravet mouse models is variable penetrance and expressivity of Dravet-like phenotypes dependent on genetic strain background (Miller et al. 2014; Rubinstein et al. 2015; Yu et al. 2006). We generated the Scn1atmKea model with heterozygous deletion of the first coding exon (abbreviated Scn1a+/−) (Miller et al. 2014). Phenotype severity was highly dependent on strain background. On the 129S6/SvEvTac (129) strain, Scn1a+/− mice did not develop epilepsy and lived a normal lifespan. In contrast, when crossed with C57BL/6J (B6) mice, the resulting [B6 × 129]F1.Scn1a+/− mice recapitulated core features of Dravet syndrome, including spontaneous seizures and epilepsy, and a high incidence of SUDEP-like death in the first month of life (Miller et al. 2014). We previously performed genetic mapping to identify Dravet survival modifier (Dsm) loci influencing strain-dependent survival of Scn1a+/− mice. Five Dsm loci were localized to mouse chromosomes 5, 7, 8 and 11 (Miller et al. 2014). Dsm1 and Dsm4 mapped to an overlapping region on chromosome 5 in both 129 and B6 backcrosses (Miller et al. 2014). Further fine mapping and candidate gene analyses of Dsm1/4 locus on chromosome 5 identified and validated Gabra2 as a modifier gene (Hawkins et al. 2021, 2016; Nomura et al. 2019). In the current study, we used a similar interval-specific congenic (ISC) mapping approach to refine Dsm2 and Dsm3 on chromosomes 7 and 8, respectively. In addition, we performed candidate gene analysis within the refined intervals to nominate putative modifier genes that may influence strain-dependent survival of Scn1a+/− Dravet mice.
Methods
Mice (NCBI Taxon ID 10090)
Scn1atm1Kea mice (RRID:MMRRC 037107-JAX) are maintained as an isogenic strain by continued backcrossing to 129S6/SvEvTac inbred mice (129SVE, Taconic Biosciences, Germantown, NY, USA), and abbreviated herein as 129.Scn1a+/− (Miller et al. 2014). C57BL/6J (B6) inbred mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA; RRID: IMSR_JAX: 000664). Mice were maintained in a Specific Pathogen Free (SPF) barrier facility with a 14:10 light:dark cycle and access to food and water ad libitum. Animal care and experimental procedures were approved by the Northwestern University Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The principles outlined in the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines were considered when planning experiments (Percie du Sert et al. 2020).
Interval specific congenic (ISC) lines
ISC lines were generated by crossing 129SvEvTac (129) males with B6 females, and then successively crossing to B6 to generate congenic lines with 129-derived alleles on the chromosome of interest on a B6 background. Separate series of ISC strains were developed for Dsm2 (designated as B6.129-ISC7A through B6.129-ISC7H) on chromosome 7, and for Dsm3 (designated as B6.129-ISC8A through B6.129-ISC8G) on chromosome 8. At each generation mice were genotyped for microsatellite markers in the region of interest and mice retaining 129 alleles were propagated. Whole genome SNP genotyping was performed at generations N4 and N7 to select breeders with low percentages of 129 in the rest of the genome. All lines were crossed to B6 for ≥ N8 generations prior to any experiments.
Genotyping
DNA was prepared from tail biopsies obtained at approximately 2-weeks of age (Gentra Puregene Mouse Tail Kit, Qiagen, Valencia, CA, USA). Scn1a genotype was determined as previously described (Kearney et al. 2022). Microsatellite genotypes were determined by analysis of PCR products on 7% nondenaturing polyacrylamide gels. SNP genotypes were determined using the mini Mouse Universal Genotyping Array (miniMUGA) (Transnetyx, Cordova, TN). Breakpoints for ISC lines were refined using miniMUGA analysis at ≥ N8.
Phenotyping
Female B6.129-ISC7 or B6.129-ISC8 mice were bred with heterozygous 129.Scn1a+/− males to generate F1 offspring carrying homozygous 129/129 alleles or heterozygous 129/B6 alleles in the Dsm2 or Dsm3 interval, respectively. This breeding scheme neutralizes confounding parent-of-origin effects for imprinted genes, a number of which reside on mouse chromosome 7 (Morison and Reeve 1998). At P12-14, mice were ear-tagged, tail biopsied and genotyped. Mice were weaned at P19-21 into standard vivarium cages containing 4–5 mice of the same sex/age and monitored for survival to 8 weeks age. Over the monitoring period, mice were checked daily for general health. Any visibly unhealthy mouse (e.g., underweight, dehydrated, poorly groomed, or immobile) was euthanized and excluded (rare) because the focus of the study was sudden and unexpected death in otherwise healthy appearing Scn1a+/− mice (SUDEP-like). Subjects surviving beyond P56 were censored for survival analysis because they reached the predetermined end of the study period. Dsm2 and Dsm3 were analyzed separately with their respective ISC strains. Survival was compared between groups using Kaplan–Meier analysis with hazard ratios and P-values determined by LogRank Mantel–Cox tests. To minimize false positives due to multiple testing, false discovery rate (FDR)-adjusted P-values were calculated and significance thresholds were determined by the Benjamini‐Hochberg method (Benjamini and Hochberg 1995)(q < 0.1). Group sizes were determined by data simulations using data from our prior survival studies (Hawkins et al. 2016; Kearney et al. 2022; Miller et al. 2014).
Candidate gene analysis
Candidate gene sets were extracted from the Mus musculus GRCm39 reference genome using the Ensembl BioMart tool (Martin et al. 2023). The subset of genes expressed in brain were defined using the MGI Gene eXpression Database (Baldarelli et al. 2021). Differential expression of candidate genes between 129 and B6 or F1 was assessed using two RNA-Seq datasets that we previously reported: (1) B6 and 129 forebrain (Hawkins et al. 2016); and (2) F1 and 129 hippocampus (Hawkins et al. 2019) (NCBI GEO GSE112627). Coding sequence changes between strains were derived from our previously reported whole genome re-sequencing of 129S6/SvEvTac (NCBI SRA PRJNA817075) aligned to the C57BL/6 J reference sequence (Kearney et al. 2022). Gene- and filter-based annotation was performed using SnpEff and variant effect predictions were assessed using the Ensembl Variant Effect Predictor (VEP) (Cingolani et al. 2012; McLaren et al. 2016). SIFT and PolyPhen2 were used for predictions of missense variant effects on proteins (Adzhubei et al. 2010; Vann et al. 2018). Variants are reported with dbSNP Reference SNP (rs) accession numbers whenever possible and genomic positions are based on the Mus musculus GRCm39 reference genome. Interval specific variant summaries are reported in Supplementary Table S1.
Results
We previously reported low resolution mapping of Dsm loci that influenced strain-dependent survival of Scn1a+/− mice (Miller et al. 2014). In that report, we demonstrated that 129 alleles conferred risk at Dsm2 on chromosome 7, whereas 129 alleles were protective at Dsm3 on chromosome 8 (Miller et al. 2014). To refine the map intervals for these loci, we generated ISC lines carrying 129-derived alleles in the regions of interest on an otherwise B6 background. Separate series of lines were generated and analyzed for Dsm2 on chromosome 7 (designated ISC7-letter) and Dsm3 on chromosome 8 (designated ISC8-letter). For each line, ISC females were crossed to 129.Scn1a+/− males and survival was monitored to 8 weeks of age. Survival was then compared between offspring carrying homozyogous (129/129) alleles in the ISC region or heterozygous (129/B6) F1 controls. Based on our initial mapping, we expected that 129 homozygosity would result in worse survival for modifier-containing intervals on chromosome 7 and improved survival for modifier-containing intervals on chromosome 8 (Miller et al. 2014).
Dsm2 fine mapping and candidate gene analysis
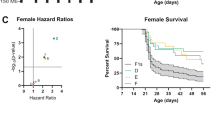
To map Dsm2, we used eight ISC lines carrying 129-derived alleles across the interval on chromosome 7 (Fig. 1A). The region on chromosome 7 is complex and likely has multiple contributing loci, similar to our previous report of Dsm5 on mouse chromosome 11 (Kearney et al. 2022). Line ISC7-H confers significant risk, while lines ISC7-C, ISC7-F, and ISC7-B trend toward moderate risk (Fig. 1B, C) (Table 1). We interpret this as there being proximal and distal risk alleles that synergize in line ISC7-H (Fig. 1A). Potential protective contributions from distal regions of the mapped interval may attenuate risk in lines ISC7-C, ISC7-B, ISC7-G, and ISC7-A (Fig. 1A). The map intervals of interest for Dsm2 are 25.3–34.1 Mb (Dsm2a) and 45.9–52.9 Mb (Dsm2b) on chromosome 7.
Fine mapping of Dsm2 with ISC strains. A Dsm2 lines have varying 129-derived intervals (red) on a congenic B6 background (grey). B6.129-Dsm2 ISC7 lines were crossed with 129.Scn1a+/− mice and survival of Scn1a+/− offspring was monitored to 8 weeks of age. B Hazard ratios for all Dsm2 ISC lines compared to F1 controls plotted against −log10(P-values) determined by Mantel-Cox LogRank test (n = 18–24 per line). C Kaplan Meier survival plots are shown for each ISC7 line (red) compared to F1.Scn1a+/− controls (black). Shaded area represents 95% confidence interval for F1. Scn1a+/− controls
At the current resolution, the Dsm2a region contains 224 known protein coding genes and 36 noncoding RNA genes, and will require additional refinement to support candidate gene prioritization. In contrast, the Dsm2b interval is tractable and contains 51 known protein coding genes and 5 known noncoding RNA genes. Among the 51 protein coding genes in Dsm2b, 32 are expressed in brain (Table 2). We evaluated the brain expressed candidates for evidence of differential gene expression (DEG) in our existing mRNA-seq datasets (Hawkins et al. 2019; Miller et al. 2014). We found a total of five genes with evidence of differential expression (Table 2). There were three DEGs when comparing F1.Scn1a+/− mice with or without recent seizures (Ldha, Ptpn5, Sergef) (Fig. 2A, B, C), and two DEGs when comparing the strains within genotypes (Ano5, Nav2) (Fig. 2D, E). Within the Dsm2b interval, there are 9084 SNPs and 3062 indel variants between 129 and B6, with more than half located in intergenic regions (Supplementary Tables S1, S2, S3). In terms of consequential coding sequence differences, we identified six genes in the refined Dsm2b interval with predicted missense or in-frame indel variants (Dbx1, Myod1, Nav2, Prmt3, Sergef, Slc6a5) (Table 2). Most missense variants were predicted to be tolerated or benign; however, variants in Myod1 (rs13472315) and Nav2 (rs32337268) were predicted to be probably damaging and possibly damaging/deleterious, respectively (Supplementary Table S4). Additionally, Dbx1 has an in-frame deletion of 2 amino acids in 129 versus B6, and Nav2 has an in-frame insertion that results in modest expansion of a polyglutamine repeat in 129. Among the nine genes with coding sequence or expression differences, six had a previous association with epilepsy and/or seizures based on literature and database searches (Dbx1, Ldha, Nav2, Prmt3, Ptpn5, Slc6a5) (Fig. 2F) (Table 2).
Analysis of Dsm2 positional candidate genes. A–E Differential expression of candidate modifier genes based on analysis of our previously published RNA-seq datasets (Hawkins et al. 2019, 2016). A–C Comparing F1.Scn1a+/− mice with or without seizures in the 24 h preceding tissue collection, three genes were differentially expressed in hippocampus, Ldha (A), Ptpn5 (B), and Sergef (C). D Ano5 had lower hippocampal expression in F1.Scn1a+/− compared to 129.Scn1a+/− mice at postnatal day 14 (P14). E Nav2 had lower hippocampal expression in wild-type (WT) 129 versus F1 mice at postnatal day 24 (P24). F Summary of strain-dependent differences in candidate genes and their association with seizures and/or epilepsy. Six genes had strain-dependent differences and an existing biological link with seizures or epilepsy. ***FDR-adjusted p < 0.0001, **FDR-adjusted p < 0.003, *FDR-adjusted p < 0.07 (from DeSeq2 reported in (Hawkins et al. 2019))
Dsm3 fine mapping and candidate gene analysis
For fine mapping of Dsm3, we used eight ISC lines with varying 129-derived intervals in the Dsm3 region on chromosome 8 (Fig. 3A). Lines ISC8-B, ISC8-A and ISC8-E conferred improved survival, while other lines did not (Fig. 3B, C) (Table 3). Based on this, we localized the map interval to 66.2–68.8 Mb on chromosome 8. Genomic variants between 129 and B6 in the Dsm3 interval include 1192 SNPs and 547 indels, with 80% located in intergenic regions (Supplementary Tables S1, S5, S6).
Fine mapping of Dsm3 with ISC strains. A Dsm3 lines have varying 129-derived chromosome 8 intervals (blue) on a congenic B6 background (grey). B6.129-Dsm3 ISC8 lines were crossed with 129.Scn1a+/− mice and survival of Scn1a+/− offspring was monitored to 8 weeks of age. B Hazard ratios for all Dsm3 ISC lines compared to F1 controls plotted against -log10(P-values) determined by Mantel-Cox LogRank test (n = 19–24 per line). C Kaplan Meier survival plots are shown for each ISC8 line (blue) compared to F1.Scn1a+/− controls (black). Shaded area represents 95% confidence interval for F1.Scn1a+/− controls
The refined Dsm3 interval contains 11 known protein coding genes. Of those, seven have confirmed expression in the brain. None of the brain expressed candidate genes have evidence of strain-dependent differences in gene expression, while two have missense coding sequence differences (Psd3, Sh2d4a) (Table 4). Psd3, encoding pleckstrin and Sec7 domain containing 3, has two missense variants between the strains that are each individually predicted to be tolerated/benign (Supplementary Table S4); however, they are in relatively close proximity and the combined effect of both substitutions is unknown. Sh2d4a, encoding SH2 domain containing 4A, has a missense variant that is predicted to be deleterious (Supplementary Table S4). Based on the literature and database searches, there is association with seizures or epilepsy for five of the brain expressed genes (Marchf1, Tma16, Npy1r, Npy5r, Psd3). Presently, Psd3 is the only gene in the interval with evidence of strain-dependent differences and a biological association with seizures or epilepsy.
Discussion
In the current study, we used ISC mapping to refine two Dravet syndrome modifier loci, Dsm2 on mouse chromosome 7 and Dsm3 on chromosome 8. Chromosome 7 was complex, with additive effects that suggested the contribution of at least two loci designated as Dsm2a and Dsm2b. Within the refined Dsm2b and Dsm3 intervals we identified a number of modifier gene candidates that were prioritized based on (1) evidence of brain expression, (2) evidence of strain-dependent differences in expression or coding sequence, and (3) a plausible biological association with seizures/epilepsy. High priority candidates will be evaluated empirically in future studies to validate their modifier potential.
Among the Dsm2b genes, six were deemed strong candidates as they met our three levels of evidence. We further prioritized the genes based on strength of the biological association with seizures or epilepsy. Nav2, Ptpn5, Ldha, and Dbx1 are deemed higher priority as they have a direct biological association with seizures or epilepsy, while Prmt3 and Slc6a5 have an indirect association. For Dsm3, there was a single gene, Psd3, that met our three levels of evidence for a strong candidate. All strong candidates are discussed briefly below.
Nav2 encodes Neuron navigator 2, a cytoskeletal associated protein important for cortical neuron migration and axon growth (Powers et al. 2022). Bi-allelic loss-of-function of NAV2 was reported to cause a neurodevelopmental disorder with a complex brain malformation (Accogli et al. 2023). Disruption of the Drosophila ortholog (Sickie) results in hyperthermia-induced seizures and lethality (McNeill et al. 2011).
Ptpn5 encodes Protein tyrosine phosphatase, non-receptor type 5, also known as Striatal-Enriched protein tyrosine Phosphatase (STEP). Deletion of Ptpn5 in mice results in resistance to seizures induced by pilocarpine and attenuates audiogenic seizures in fragile X syndrome mice (Briggs et al. 2011; Chatterjee et al. 2018). In addition, pharmacological inhibition of STEP protects mice from seizures induced by kainic acid (Walters et al. 2022).
Ldha encodes lactate dehydrogenase A, a key enzyme in the astrocyte-neuron lactate shuttle. The lactate shuttle and lactate dehydrogenase (LDH) are inhibited by the ketogenic diet and stiripentol (Sada et al. 2015). Both are recommended therapies according to the Dravet syndrome International Consensus Panel, with stiripentol recommended as a second line treatment and ketogenic diet as a fourth line treatment (Wirrell et al. 2022). Furthermore, intrahippocampal antisense oligodeoxynucleotide knockdown of Ldha in mice suppressed high voltage spikes following kainic acid administration (Sada et al. 2015).
Dbx1 encodes Developing brain homeobox 1, a transcription factor critical for interneuron differentiation, including Cajal Retzius cells in the cortex and brainstem pre-Bötzinger complex interneurons (Akins et al. 2017; Powers et al. 2022; Vann et al. 2018). Interestingly, the pre-Bötzinger complex is a critical nucleus for generating respiratory rhythms and has been implicated in SUDEP (Akins et al. 2017; George et al. 2023; Xia et al. 2016).
Prmt3 encodes Protein arginine methyltransferase 3 that has been shown to methylate the cardiac NaV1.5 sodium channel and modulate its function (Beltran-Alvarez et al. 2013). Similarly, NaV1.2 function was modulated by Prmt8-mediated arginine methylation in vitro, while kainic acid-induced seizures in vivo resulted in elevated arginine methylation of NaV1.2 at three sites (Baek et al. 2014). Together, these observations raise the possibility of arginine methylation of NaV1.1 as a dynamic post-translation modification that could be mediated by Prmt3, which is expressed in brain.
Slc6a5 encodes a presynaptic glycine transporter. Pathogenic variants in human SLC6A5 result in hyperekplexia characterized by exaggerated startle response and life-threatening apneas that can result in sudden death in infancy (Rees et al. 2006). Although seizures are not a prominent feature of hyperekplexia, the strong association of SLC6A5 with paroxysmal apneas suggests the possibility of enhanced SUDEP risk in the context of pre-existing epilepsy.
Psd3 encodes pleckstrin and Sec7 domain containing 3, a guanine nucleotide exchange factor for ARF6 whose activity is critical for GABAergic synapse development (Kim et al. 2020; Sakagami et al. 2006). Knockdown of Arf6 in mice results in enhanced seizure susceptibility, while dysregulation of ARF6 via IQSEC2 pathogenic variants are associated with epilepsy, intellectual disability, and autism (Brant et al. 2021; Kim et al. 2020). Although we did not observe differential expression of Psd3 in our transcriptomic datasets, a proteomics study of another Dravet mouse model (Scn1aA1873V) reported lower hippocampal expression of Psd3 in Dravet versus WT mice at 4 weeks of age (Miljanovic et al. 2021).
The Dsm2a region is still large and requires additional refinement for efficient candidate gene analysis; however, it has not escaped our notice Scn1b is in this interval. Scn1b encodes the voltage-gated sodium channel beta subunit 1, which regulates NaV1.1 expression, localization, and channel gating (O’Malley and Isom 2015). It has already been reported that Scn1a+/− mice exhibited a deficit in Scn1b expression, and that AAV-Scn1b administration targeting GABAergic neurons improved survival in Scn1a+/− mice (Niibori et al. 2020). This provides support for Scn1b as a modifier of the Scn1a+/− survival phenotype. However, because the mapped interval on proximal chromosome 7 is still large, the possibility of other contributing genes remains open.
Potential limitations of our study include the following. First, the Dsm2 fine mapping data support the possibility of additive modifier genes on chromosome 7. This possibility could be empirically assessed with ISC lines that separate the proximal and distal intervals if the isolated effects sizes are large enough. However, in almost four years of breeding, recombination within this critical interval has not yet occurred in our colony that relies on natural recombination. It may be possible to engineer the desired recombination in the future. Recently, it was demonstrated that CRISPR/Cas9 editing can be utilized to circumvent recombination suppression in nematodes (Xie et al. 2023). Secondly, although we have evidence that some candidate genes are differentially expressed, absence of evidence should not be over-interpreted. Surveying expression of biologically plausible candidates across different brain regions and/or time points may reveal differences that were not captured in our existing datasets and could potentially elevate candidates that currently lack evidence of a strain-dependent difference. Notably, there are many noncoding variants both in inter- and intra-genic regions within each interval that could potentially modulate gene regulation. Finally, absence of an existing association with epilepsy or seizures does not dismiss potential candidates; however, additional evidence is required to support a modifier role. Future studies may be necessary to gather additional evidence if high priority candidates fail to validate.
Conclusion
Variable expressivity is common among individuals with Dravet syndrome due to SCN1A haploinsufficiency, supporting that genetic modifiers may contribute to clinical severity. It is challenging to discover modifier genes in humans in the context of rare disorders; therefore, we used the Scn1a+/− Dravet mouse model as a tractable system for identification of modifier loci and candidate genes. Identifying modifier genes that influence clinical severity will advance our understanding of disease mechanisms and suggest potential targets for therapeutic intervention.
Data availability
Genomic and transcriptomic datasets analyzed during the current study are available in the NCBI GEO repository [GSE112627] and SRA repository [PRJNA817075]. Other datasets are available from [https://doi.org/10.18131/yx5ys-tbh43].
Code availability
Not applicable.
References
Accogli A, Lu S, Musante I, Scudieri P, Rosenfeld JA, Severino M, Baldassari S, Iacomino M, Riva A, Balagura G, Piccolo G, Minetti C, Roberto D, Xia F, Razak R, Lawrence E, Hussein M, Chang EY, Holick M, Calì E, Aliberto E, De-Sarro R, Gambardella A, UD Network, SS Group, Emrick L, McCaffery PJA, Clagett-Dame M, Marcogliese PC, Bellen HJ, Lalani SR, Zara F, Striano P, Salpietro V (2023) Loss of neuron navigator 2 impairs brain and cerebellar development. Cerebellum 22:206–222
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249
Akins VT, Weragalaarachchi K, Picardo MCD, Revill AL, Del Negro CA (2017) Morphology of Dbx1 respiratory neurons in the preBötzinger complex and reticular formation of neonatal mice. Sci Data 4:170097
Baek JH, Rubinstein M, Scheuer T, Trimmer JS (2014) Reciprocal changes in phosphorylation and methylation of mammalian brain sodium channels in response to seizures. J Biol Chem 289:15363–15373
Baldarelli RM, Smith CM, Finger JH, Hayamizu TF, McCright IJ, Xu J, Shaw DR, Beal JS, Blodgett O, Campbell J, Corbani LE, Frost PJ, Giannatto SC, Miers DB, Kadin JA, Richardson JE, Ringwald M (2021) The mouse gene expression database (GXD): 2021 update. Nucleic Acids Res 49:D924-d931
Beltran-Alvarez P, Espejo A, Schmauder R, Beltran C, Mrowka R, Linke T, Batlle M, Pérez-Villa F, Pérez GJ, Scornik FS, Benndorf K, Pagans S, Zimmer T, Brugada R (2013) Protein arginine methyl transferases-3 and -5 increase cell surface expression of cardiac sodium channel. FEBS Lett 587:3159–3165
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 57:289–300
Brant B, Stern T, Shekhidem HA, Mizrahi L, Rosh I, Stern Y, Ofer P, Asleh A, Umanah GKE, Jada R, Levy NS, Levy AP, Stern S (2021) IQSEC2 mutation associated with epilepsy, intellectual disability, and autism results in hyperexcitability of patient-derived neurons and deficient synaptic transmission. Mol Psychiatry 26:7498–7508
Briggs SW, Walker J, Asik K, Lombroso P, Naegele J, Aaron G (2011) STEP regulation of seizure thresholds in the hippocampus. Epilepsia 52:497–506
Chatterjee M, Kurup PK, Lundbye CJ, Hugger Toft AK, Kwon J, Benedict J, Kamceva M, Banke TG, Lombroso PJ (2018) STEP inhibition reverses behavioral, electrophysiologic, and synaptic abnormalities in Fmr1 KO mice. Neuropharmacology 128:43–53
Cingolani P, Platts A, le Wang L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (austin) 6:80–92
Cooper MS, McIntosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, Ganesan V, Gill D, Kivity S, Lerman-Sagie T, McLellan A, Pelekanos J, Ramesh V, Sadleir L, Wirrell E, Scheffer IE (2016) Mortality in Dravet syndrome. Epilepsy Res 128:43–47
Darra F, Battaglia D, Dravet C, Patrini M, Offredi F, Chieffo D, Piazza E, Fontana E, Olivieri G, Turrini I, DallaBernardina B, Granata T, Ragona F (2019) Dravet syndrome: early electroclinical findings and long-term outcome in adolescents and adults. Epilepsia 60(Suppl 3):S49-s58
de Lange IM, Mulder F, van’t Slot R, Sonsma ACM, van Kempen MJA, Nijman IJ, Ernst RF, Knoers N, Brilstra EH, Koeleman BPC (2020) Modifier genes in SCN1A-related epilepsy syndromes. Mol Genet Genomic Med 8:e1103
Depienne C, Trouillard O, Saint-Martin C, Gourfinkel-An I, Bouteiller D, Carpentier W, Keren B, Abert B, Gautier A, Baulac S, Arzimanoglou A, Cazeneuve C, Nabbout R, LeGuern E (2009) Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet 46:183–191
Depienne C, Trouillard O, Gourfinkel-An I, Saint-Martin C, Bouteiller D, Graber D, Barthez-Carpentier MA, Gautier A, Villeneuve N, Dravet C, Livet MO, Rivier-Ringenbach C, Adam C, Dupont S, Baulac S, Héron D, Nabbout R, Leguern E (2010) Mechanisms for variable expressivity of inherited SCN1A mutations causing Dravet syndrome. J Med Genet 47:404–410
Dravet C (2011) The core Dravet syndrome phenotype. Epilepsia 52(Suppl 2):3–9
Dravet C, Oguni H (2013) Dravet syndrome (severe myoclonic epilepsy in infancy). Handb Clin Neurol 111:627–633
Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O (2005) Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol 95:71–102
Feng T, Makiello P, Dunwoody B, Steckler F, Symonds JD, Zuberi SM, Dorris L, Brunklaus A (2024) Long-term predictors of developmental outcome and disease burden in SCN1A-positive Dravet syndrome. Brain Commun 6(1):fcae004
Gallagher D, Pérez-Palma E, Bruenger T, Ghanty I, Brilstra E, Ceulemans B, Chemaly N, de Lange I, Depienne C, Guerrini R, Mei D, Møller RS, Nabbout R, Regan BM, Schneider AL, Scheffer IE, Schoonjans AS, Symonds JD, Weckhuysen S, Zuberi SM, Lal D, Brunklaus A (2024) Genotype-phenotype associations in 1018 individuals with SCN1A-related epilepsies. Epilepsia 65(4):1046–1059
George AG, Farrell JS, Colangeli R, Wall AK, Gom RC, Kesler MT, Rodriguez de la Hoz C, Villa BR, Perera T, Rho JM, Kurrasch D, Teskey GC (2023) Sudden unexpected death in epilepsy is prevented by blocking postictal hypoxia. Neuropharmacology 231:109513
Goldberg-Stern H, Aharoni S, Afawi Z, Bennett O, Appenzeller S, Pendziwiat M, Kuhlenbäumer G, Basel-Vanagaite L, Shuper A, Korczyn AD, Helbig I (2014) Broad phenotypic heterogeneity due to a novel SCN1A mutation in a family with genetic epilepsy with febrile seizures plus. J Child Neurol 29:221–226
Guerrini R, Cellini E, Mei D, Metitieri T, Petrelli C, Pucatti D, Marini C, Zamponi N (2010) Variable epilepsy phenotypes associated with a familial intragenic deletion of the SCN1A gene. Epilepsia 51:2474–2477
Hammer MF, Ishii A, Johnstone L, Tchourbanov A, Lau B, Sprissler R, Hallmark B, Zhang M, Zhou J, Watkins J, Hirose S (2017) Rare variants of small effect size in neuronal excitability genes influence clinical outcome in Japanese cases of SCN1A truncation-positive Dravet syndrome. PLoS ONE 12:e0180485
Hawkins NA, Zachwieja NJ, Miller AR, Anderson LL, Kearney JA (2016) Fine mapping of a Dravet syndrome modifier locus on mouse chromosome 5 and candidate gene analysis by RNA-seq. PLoS Genet 12:e1006398
Hawkins NA, Calhoun JD, Huffman AM, Kearney JA (2019) Gene expression profiling in a mouse model of Dravet syndrome. Exp Neurol 311:247–256
Hawkins NA, Nomura T, Duarte S, Barse L, Williams RW, Homanics GE, Mulligan MK, Contractor A, Kearney JA (2021) Gabra2 is a genetic modifier of Dravet syndrome in mice. Mamm Genome 32:350–363
Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA (2013) Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest 123:1798–1808
Kearney JA (2011) Genetic modifiers of neurological disease. Curr Opin Genet Dev 21:349–353
Kearney JA, Copeland-Hardin LD, Duarte S, Zachwieja NA, Eckart-Frank IK, Hawkins NA (2022) Fine mapping and candidate gene analysis of a dravet syndrome modifier locus on mouse chromosome 11. Mamm Genome 33:565–574
Kim H, Jung H, Jung H, Kwon SK, Ko J, Um JW (2020) The small GTPase ARF6 regulates GABAergic synapse development. Mol Brain 13:2
Martin FJ, Amode MR, Aneja A, Austine-Orimoloye O, Azov AG, Barnes I, Becker A, Bennett R, Berry A, Bhai J, Bhurji SK, Bignell A, Boddu S, BrancoLins PR, Brooks L, Ramaraju SB, Charkhchi M, Cockburn A, Da Rin FL, Davidson C, Dodiya K, Donaldson S, El Houdaigui B, El Naboulsi T, Fatima R, Giron CG, Genez T, Ghattaoraya GS, Martinez JG, Guijarro C, Hardy M, Hollis Z, Hourlier T, Hunt T, Kay M, Kaykala V, Le T, Lemos D, Marques-Coelho D, Marugán JC, Merino GA, Mirabueno LP, Mushtaq A, Hossain SN, Ogeh DN, Sakthivel MP, Parker A, Perry M, Piližota I, Prosovetskaia I, Pérez-Silva JG, Salam AIA, Saraiva-Agostinho N, Schuilenburg H, Sheppard D, Sinha S, Sipos B, Stark W, Steed E, Sukumaran R, Sumathipala D, Suner MM, Surapaneni L, Sutinen K, Szpak M, Tricomi FF, Urbina-Gómez D, Veidenberg A, Walsh TA, Walts B, Wass E, Willhoft N, Allen J, Alvarez-Jarreta J, Chakiachvili M, Flint B, Giorgetti S, Haggerty L, Ilsley GR, Loveland JE, Moore B, Mudge JM, Tate J, Thybert D, Trevanion SJ, Winterbottom A, Frankish A, Hunt SE, Ruffier M, Cunningham F, Dyer S, Finn RD, Howe KL, Harrison PW, Yates AD, Flicek P (2023) Ensembl 2023. Nucleic Acids Res 51:D933-d941
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F (2016) The ensembl variant effect predictor. Genome Biol 17:122
McNeill EM, Klöckner-Bormann M, Roesler EC, Talton LE, Moechars D, Clagett-Dame M (2011) Nav2 hypomorphic mutant mice are ataxic and exhibit abnormalities in cerebellar development. Dev Biol 353:331–343
Miljanovic N, Hauck SM, van Dijk RM, Di Liberto V, Rezaei A, Potschka H (2021) Proteomic signature of the Dravet syndrome in the genetic Scn1a-A1783V mouse model. Neurobiol Dis 157:105423
Miller AR, Hawkins NA, McCollom CE, Kearney JA (2014) Mapping genetic modifiers of survival in a mouse model of Dravet syndrome. Genes Brain Behav 13:163–172
Morison IM, Reeve AE (1998) A catalogue of imprinted genes and parent-of-origin effects in humans and animals. Hum Mol Genet 7:1599–1609
Nabbout R, Gennaro E, DallaBernardina B, Dulac O, Madia F, Bertini E, Capovilla G, Chiron C, Cristofori G, Elia M, Fontana E, Gaggero R, Granata T, Guerrini R, Loi M, La Selva L, Lispi ML, Matricardi A, Romeo A, Tzolas V, Valseriati D, Veggiotti P, Vigevano F, Vallée L, DagnaBricarelli F, Bianchi A, Zara F (2003) Spectrum of SCN1A mutations in severe myoclonic epilepsy of infancy. Neurology 60:1961–1967
Niibori Y, Lee SJ, Minassian BA, Hampson DR (2020) Sexually divergent mortality and partial phenotypic rescue after gene therapy in a mouse model of Dravet syndrome. Hum Gene Ther 31:339–351
Nomura T, Hawkins NA, Kearney JA, George AL Jr, Contractor A (2019) Potentiating α(2) subunit containing perisomatic GABA(A) receptors protects against seizures in a mouse model of Dravet syndrome. J Physiol 597:4293–4307
O’Malley HA, Isom LL (2015) Sodium channel β subunits: emerging targets in channelopathies. Annu Rev Physiol 77:481–504
Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K (2007) Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 27:5903–5914
Osaka H, Ogiwara I, Mazaki E, Okamura N, Yamashita S, Iai M, Yamada M, Kurosawa K, Iwamoto H, Yasui-Furukori N, Kaneko S, Fujiwara T, Inoue Y, Yamakawa K (2007) Patients with a sodium channel alpha 1 gene mutation show wide phenotypic variation. Epilepsy Res 75:46–51
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ Open Sci 4(1):e100115
Powers RM, Hevner RF, Halpain S (2022) The neuron navigators: structure, function, and evolutionary history. Front Mol Neurosci 15:1099554
Rees MI, Harvey K, Pearce BR, Chung SK, Duguid IC, Thomas P, Beatty S, Graham GE, Armstrong L, Shiang R, Abbott KJ, Zuberi SM, Stephenson JB, Owen MJ, Tijssen MA, van den Maagdenberg AM, Smart TG, Supplisson S, Harvey RJ (2006) Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat Genet 38:801–806
Ricobaraza A, Mora-Jimenez L, Puerta E, Sanchez-Carpintero R, Mingorance A, Artieda J, Nicolas MJ, Besne G, Bunuales M, Gonzalez-Aparicio M, Sola-Sevilla N, Valencia M, Hernandez-Alcoceba R (2019) Epilepsy and neuropsychiatric comorbidities in mice carrying a recurrent Dravet syndrome SCN1A missense mutation. Sci Rep 9:14172
Rubinstein M, Westenbroek RE, Yu FH, Jones CJ, Scheuer T, Catterall WA (2015) Genetic background modulates impaired excitability of inhibitory neurons in a mouse model of Dravet syndrome. Neurobiol Dis 73:106–117
Sada N, Lee S, Katsu T, Otsuki T, Inoue T (2015) Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science (new York, N.y.) 347(6228):1362–1367
Sakagami H, Suzuki H, Kamata A, Owada Y, Fukunaga K, Mayanagi H, Kondo H (2006) Distinct spatiotemporal expression of EFA6D, a guanine nucleotide exchange factor for ARF6, among the EFA6 family in mouse brain. Brain Res 1093:1–11
Sakauchi M, Oguni H, Kato I, Osawa M, Hirose S, Kaneko S, Takahashi Y, Takayama R, Fujiwara T (2011a) Mortality in Dravet syndrome: search for risk factors in Japanese patients. Epilepsia 52(Suppl 2):50–54
Sakauchi M, Oguni H, Kato I, Osawa M, Hirose S, Kaneko S, Takahashi Y, Takayama R, Fujiwara T (2011b) Retrospective multiinstitutional study of the prevalence of early death in Dravet syndrome. Epilepsia 52:1144–1149
Strzelczyk A, Lagae L, Wilmshurst JM, Brunklaus A, Striano P, Rosenow F, Schubert-Bast S (2023) Dravet syndrome: a systematic literature review of the illness burden. Epilepsia Open 8:1256–1270
Vann NC, Pham FD, Dorst KE, Del Negro CA (2018) Dbx1 pre-Bötzinger complex interneurons comprise the core inspiratory oscillator for breathing in unanesthetized adult mice. eNeuro 5
Villas N, Meskis MA, Goodliffe S (2017) Dravet syndrome: Characteristics, comorbidities, and caregiver concerns. Epilepsy & Behavior : E&b 74:81–86
Walters JM, Kim EC, Zhang J, Jeong HG, Bajaj A, Baculis BC, Tracy GC, Ibrahim B, Christian-Hinman CA, Llano DA, Huesmann GR, Chung HJ (2022) Pharmacological inhibition of STriatal-Enriched protein tyrosine Phosphatase by TC-2153 reduces hippocampal excitability and seizure propensity. Epilepsia 63:1211–1224
Wirrell EC, Hood V, Knupp KG, Meskis MA, Nabbout R, Scheffer IE, Wilmshurst J, Sullivan J (2022) International consensus on diagnosis and management of Dravet syndrome. Epilepsia 63:1761–1777
Xia G, Pourali SP, Warner TA, Zhang CQ, Macdonald RL, Kang JQ (2016) Altered GABAA receptor expression in brainstem nuclei and SUDEP in Gabrg2(+/Q390X) mice associated with epileptic encephalopathy. Epilepsy Res 123:50–54
Xie D, Gu B, Liu Y, Ye P, Ma Y, Wen T, Song X, Zhao Z (2023) Efficient targeted recombination with CRISPR/Cas9 in hybrids of Caenorhabditis nematodes with suppressed recombination. BMC Biol 21:203
Yordanova I, Todorov T, Dimova P, Hristova D, Tincheva R, Litvinenko I, Yotovska O, Kremensky I, Todorova A (2011) One novel Dravet syndrome causing mutation and one recurrent MAE causing mutation in SCN1A gene. Neurosci Lett 494:180–183
Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA (2006) Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 9:1142–1149
Yu MJ, Shi YW, Gao MM, Deng WY, Liu XR, Chen L, Long YS, Yi YH, Liao WP (2010) Milder phenotype with SCN1A truncation mutation other than SMEI. Seizure 19:443–445
Zuberi SM, Brunklaus A, Birch R, Reavey E, Duncan J, Forbes GH (2011) Genotype-phenotype associations in SCN1A-related epilepsies. Neurology 76:594–600
Acknowledgements
We thank Levi Barse, Tyler Thenstedt, Rylie Pancoast, Kelsey Davis, and Olivia Valente for technical assistance.
Funding
This work was supported by the National Institutes of Neurological Disorders and Stroke grant R01 NS084959 (JAK).
Author information
Authors and Affiliations
Contributions
Conceptualization: NAH, JAK; Formal analysis: JAK; Investigation: NAH, NS; Methodology: NAH, JAK; Project administration: NAH, JAK; Supervision: NAH, JAK; Visualization: JAK; Writing – original draft: JAK; Writing – review & editing: NAH, JAK; Funding acquisition: JAK.
Corresponding author
Ethics declarations
Competing interest
JAK serves on the Scientific Advisory Boards of the Dravet Syndrome Foundation and FamilieSCN2A Foundation. JAK receives research funding from Praxis Precision Medicines and Neurocrine Biosciences. NAH provides paid consulting services to Takeda Pharmaceuticals. All other authors have declared that no competing interests exist.
Ethical approval
Animal care and experimental procedures were approved by the Northwestern University Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hawkins, N.A., Speakes, N. & Kearney, J.A. Fine mapping and candidate gene analysis of Dravet syndrome modifier loci on mouse chromosomes 7 and 8. Mamm Genome (2024). https://doi.org/10.1007/s00335-024-10046-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00335-024-10046-3