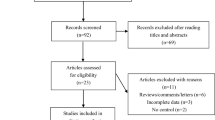
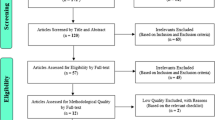
Abstract
So far, epidemiological studies have been performed to investigate the association of CDKN2A/B rs4977756 polymorphism and glioma risk. However, the results from different studies remain inconsistent. To clarify these conflicts and to quantitatively evaluate the effect of rs4977756 polymorphism on glioma risk, a meta-analysis was conducted using relevant published clinical studies about rs4977756 polymorphisms and glioma risk. Relevant studies concerning the association between rs4977756 polymorphism and risk of glioma were included in this meta-analysis. Odds ratio (OR) and 95 % confidence interval (CI) were calculated under fixed or random effects models when appropriate. Subgroup analyses were performed by race. This meta-analysis included 13 studies with a total of 8129 cases and 15,858 controls. The pooled results showed that there was an obvious association of CDKN2A/B rs4977756 polymorphism with risk of glioma in all four comparison models (dominant model/AG + GG vs. AA: OR = 1.36, 95 %CI = 1.20–1.54, p < 0.01; heterozygote comparison/AG vs. AA: OR = 1.31, 95 %CI = 1.12–1.53, p < 0.01; homozygote comparison/GG versus AA: OR = 1.49, 95 %CI = 1.36–1.64, p < 0.01; additive model/G vs. A: OR = 1.23, 95 %CI = 1.18–1.28, p < 0.01, respectively). For the subgroup analyses of ethnicities, similar results were observed in Caucasians. However, the association was not found between rs4977756 polymorphism and the risk of glioma in all models for the Asian studies. The CDKN2A/B rs4977756 polymorphism is obvious increase the risk of glioma in Caucasians. Future studies are needed to confirm the results in other ethnic populations.


Similar content being viewed by others
References
Ahmed R, Oborski MJ, Hwang M et al (2014) Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag Res 6:149–170
Bondy ML, Scheurer ME, Malmer B et al (2008) Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer 113:1953–1968
Chen H, Chen Y, Zhao Y et al (2011) Association of sequence variants on chromosomes 20, 11, and 5 (20q13.33, 11q23.3, and 5p15.33) with glioma susceptibility in a Chinese population. Am J Epidemiol 173:915–922
Di Stefano AL, Enciso-Mora V, Marie Y et al (2013) Association between glioma susceptibility loci and tumour pathology defines specific molecular etiologies. Neuro-oncology 15:542–547
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-oncology 14(Suppl 5):v1–v49
Dong YS, Hou WG, Li XL et al (2014) Genetic association of CHEK2, GSTP1, and ERCC1 with glioblastoma in the Han Chinese population. Tumour Biol 35:4937–4941
Egan KM, Thompson RC, Nabors LB et al (2011) Cancer susceptibility variants and the risk of adult glioma in a US case–control study. J Neurooncol 104:535–542
Lachance DH, Yang P, Johnson DR et al (2011) Associations of high-grade glioma with glioma risk alleles and histories of allergy and smoking. Am J Epidemiol 174:574–581
Li S, Jin T, Zhang J et al (2012) Polymorphisms of TREH, IL4R and CCDC26 genes associated with risk of glioma. Cancer Epidemiol 36:283–287
Melin B, Dahlin AM, Andersson U et al (2013) Known glioma risk loci are associated with glioma with a family history of brain tumours—a case-control gene association study. Int J Cancer J Int du Cancer 132:2464–2468
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Rajaraman P, Melin BS, Wang Z et al (2012) Genome-wide association study of glioma and meta-analysis. Hum Genet 131:1877–1888
Research Cancer Genome Atlas (2008) N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Ricard D, Idbaih A, Ducray F et al (2012) Primary brain tumours in adults. Lancet 379:1984–1996
Safaeian M, Rajaraman P, Hartge P et al (2013) Joint effects between five identified risk variants, allergy, and autoimmune conditions on glioma risk. Cancer Causes Control 24:1885–1891
Schoemaker MJ, Robertson L, Wigertz A et al (2010) Interaction between 5 genetic variants and allergy in glioma risk. Am J Epidemiol 171:1165–1173
Sharpless NE, Bardeesy N, Lee KH et al (2001) Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413:86–91
Shete S, Hosking FJ, Robertson LB et al (2009) Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 41:899–904
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Wager M, Menei P, Guilhot J et al (2008) Prognostic molecular markers with no impact on decision-making: the paradox of gliomas based on a prospective study. Br J Cancer 98:1830–1838
Wang SS, Hartge P, Yeager M et al (2011) Joint associations between genetic variants and reproductive factors in glioma risk among women. Am J Epidemiol 174:901–908
Wrensch M, Jenkins RB, Chang JS et al (2009) Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet 41:905–908
Acknowledgments
This work was generously supported by Grants from the Zhejiang Provincial Natural Science Foundation of China (Grant No. LY13H160003), Zhejiang Provincial Medicines Health Science and Technology Program Foundation of China (Grant No. 2013KYB162 and No. 2015KYB223).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Qi, X., Wan, Y., Zhan, Q. et al. Effect of CDKN2A/B rs4977756 polymorphism on glioma risk: a meta-analysis of 16 studies including 24077 participants. Mamm Genome 27, 1–7 (2016). https://doi.org/10.1007/s00335-015-9612-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-015-9612-9