Abstract
Cannabis grains are frequently reported from archaeological sites in Asia, and hypothesized centers of origins are China and Central Asia. Chinese early cannabis remains are often interpreted as evidence of hemp fabric production, in line with early textual evidence describing ritualistic hemp cloth use and hemp cultivation as a grain crop. Modern measurements on cannabis varieties show distinct sizes between fibre or oil/fibre and psychoactive varieties, the former having larger seeds on average than the latter. This paper reviews the current macro-botanical evidence for cannabis across East, Central and South Asia and builds a comparative framework based on modern cannabis seed measurements to help identify cannabis use in the past, through the metric analysis of archaeologically preserved seeds. Over 800 grains of cannabis were retrieved from the 2008 excavation of Haimenkou, Yunnan, Southwest China, dating to between 1650 and 400 bc. These are compared with other known archaeological cannabis and interpreted through the metric framework. This offers a basis for exploration of the seed morphometrics potential to infer cannabis cultivation and diversification in uses. At Haimenkou, cannabis seeds size mostly plot in the range of overlapping psychoactive/fibre types; we therefore suggest that the cannabis assemblage from Haimenkou is indicative of a crop beginning to undergo evolution from its early domesticated form towards a diversified crop specialized for alternative uses, including larger oilseed/fibre adapted varieties.
Similar content being viewed by others
Introduction
Cannabis is the source of the well-known drugs marijuana and hashish, hemp fibres used for cloth and rope, and edible oilseeds. Its domestication, early history of cultivation and diversification are presently poorly understood. Although not a staple grain today, cannabis was considered one of ancient China’s “five grains”, with millet, rice, barley, and soybean (Huang 2000). However, its presumed secondary role in the overall subsistence makes its seeds less likely to be processed in bulk and turn up in archaeobotanical assemblages when compared to cereals or pulses. Cannabis sativa (sensu lato) was probably selected early on for multiple uses as a fibre and an oilseed, as well as for medicinal/ritual drug uses. It might therefore be considered as an East Asian example comparable to the multi-use crop flax (Linum usitatissimum) that was cultivated alongside wheat and barley in early western Asia. The wild progenitor(s) of cannabis cultivars may be unknowable; today, naturalized cannabis plants that escape cultivation show wild-type characters in as little as 50 years (Small 1975) and it is posited that the native distribution area of this plant was broadly distributed in eastern Eurasia (from eastern Europe to Japan). Legal controls over the cultivation and transport of some cannabis varieties have meant that genomic datasets remain somewhat limited (see Hillig 2005). Nevertheless, there are recent taxonomic syntheses (Clarke and Merlin 2013; McPartland and Small 2020) and a growing archaeobotanical record (Jiang et al. 2016; Long et al. 2017; McPartland and Hegman 2018; Ren et al. 2019), which means we are in an improved position to deduce aspects of the domestication and differentiation process.
Although several types of cannabis remains have been found and reported archaeologically, including seeds (or achenes), seed impressions, pollen grains, fibres, textile fragments and impressions, and hemp paper, seeds have the advantage of providing the possibility of morphometric comparison, as well as attesting direct evidence for local cultivation; therefore, for this study, we focus on archaeologically reported cannabis seeds. In this paper, we present evidence for cannabis use from the Bronze Age site of Haimenkou, in northwest Yunnan Province, Southwest China (1600−400 bc), where high quantities of Cannabis sp. seeds were recovered in association with cereal remains, especially rice and millet (Xue et al. 2022). This provides a basis for consideration of the evolving uses of this plant in Yunnan and elsewhere in China, and an exploration of the potential for using seed morphometrics to infer cannabis cultivation and diversification for textile and/or oilseed use or for psychoactive/medicinal uses.
Taxonomic background and theories on the origins of cannabis
Scholars have proposed cannabis originated either in Central Asia, based on Vavilovian principles of modern distribution of highly diverse cultivated populations and archaeological pollen analyses (i.e. Vavilov and Dorofeyev 1992; Russo 2007; Long et al. 2017; McPartland et al. 2019; Rull 2022), or Northern China, due to the relative high number and frequency of early archaeological finds (Chang 1986; Wu et al. 2003; Crawford 2006). Others have proposed that modern cannabis cultigens may also derive from multiple, independent domestications (Vavilov 1926; Clarke and Merlin 2013; McPartland and Hegman 2018; Rull 2022), however recent phylogenetic analyses have posited that cannabis originated in Southwest China (Zhang et al. 2018; McPartland et al. 2019; Ren et al. 2021). To some extent these alternative theories can be linked to taxonomic uncertainty and controversy.
Cannabis belongs to the Cannabaceae family, comprising ten extant genera and about 170 species in the Old World (APG 2003), the others being hops (Humulus), and according to recent phylogenetics, trees including hackberries (Celtis L.), Aphananthe Planch., Chaetachme Planch., Gironniera Gaudich., Lozanella Greenm., Pteroceltis Maxim., Trema Lour., and Parasponia Miq. (some scholars group Parasponia with Trema; see Simpson 2010; Kovalchuk et al. 2020). Many botanical sources follow the taxonomy of Small and Cronquist (1976), more recently (Small 2017) updated considering genetic studies, which recognize just one species, Cannabis sativa L. According to the monotypic view, C. sativa is further divided into ssp. sativa var. spontanea, representing all wild and weedy varieties, ssp. sativa, representing all hemp fibre and oilseed cultivars, and ssp. indica, representing all cultivars grown primarily for psychoactive properties (see also McPartland 2018, 2020; McPartland and Small 2020). Other scholars instead argue for three separate species including C. sativa L., C. indica Lam., and C. ruderalis (Hillig 2004; Sawler et al. 2015; Clarke and Merlin 2013; Henry et al. 2020). The monotypic vs. polytypic view of Cannabis taxonomy is highly debated among scholars, however, the ICN Code (Turland et al. 2018) recognizes just one species and therefore we follow this view and outline further details about relevant subspecies and varieties below.
-
1.
Cannabis sativa L. (sensu strictu) includes both wild and domesticated forms.
-
2.
C. sativa ssp. sativa var. spontanea Vav. (syn. C. ruderalis Janisch), the narrowleaf hemp-type taxon that includes extant European wild-like varieties. In general seeds of these plants are smaller in size compared to domesticated plants, and are expected to have natural seed shattering, where the achene is detached from the seed through the formation of an abscission zone, causing the seeds to have an elongated tapered base, and prominent abscission zone, which should make it archaeobotanically distinct from domesticated forms, but de Candolle (1885) reported the presence of this type of cannabis in the South Caspian region, among other areas. This taxon likely includes feral populations, and feral hybrids resulting from introgression between sativa and indica cultivars, and thus its distribution may be the product of recent anthropogenically facilitated gene flow (see Clarke and Merlin 2013, p. 317).
-
3.
C. sativa ssp. sativa, narrowleaf hemp cultivars, mainly grown for fibre production, traditionally in Eastern Europe.
-
4.
C. sativa var. chinensis (Delile) DeBeaux. Broadleaf hemp cultivars, an East Asian textile crop, and oilseed varieties. This is hypothesized to have been selected from ssp. indica for larger seeds and/or taller plants, and generally lower THC production.
-
5.
C. sativa ssp. indica var. asperrima (Regel) McPart. & Small (syn. C. indica var. kafiristanica Vav.), the narrowleaf drug-type plants from Central Asia (McPartland and Small 2020); this variety was first described by Vavilov in the 1930s on the basis of weedy material in eastern Afghanistan. This wild taxon has significant THC production, and it is suggested to have expanded in the post-glacial period out of refugia in southwestern China, e.g. Hengduan Mountains and Yungui Plateau, i.e. Sichuan, Yunnan, Guizhou (Clarke and Merlin 2013, p. 325). This Post-Pleistocene expansion would have brought this species northward, to a point where it extended over much of northeast Asia in general. While an Indian refugium seems plausible no pollen evidence has yet been recorded to support this.
-
6.
C. sativa ssp. indica var. indica (Lam.) Persoon (syn. C. indica ssp. indica (Lam.) Clarke and Merlin). Narrowleaf drug cultivars, including Indian “ganja”. Clarke and Merlin (2013) postulated that these may be the original eastern Asia domesticated form, which lost seed shattering and the basal seed caruncle. C. sativa ssp. indica var. afghanica (Vav.) McPart. & E.Small. Broadleaf drug cultivars, Central Asian hashish, used to produce drug resin. Selected for more vegetative growth and high THC (tetrahydrocannabinol, is the main psychoactive component).
-
7.
C. sativa ssp. indica var. himalayensis (Cazzuola) McPart. & E.Small. Narrow-leaf drug type found in South Asia, especially the Himalayas (McPartland and Small 2020), typically used for hashish, seldom for seed oil.
The above taxonomy is linked to a set of evolutionary hypotheses in which wild populations were already structured into higher and lower THC varieties and inversely correlated levels of CBD (cannabidiol, which has a calming effect and medicinal uses in pain relief). For example, traditional varieties of hemp across China, are reported to range from 0.02 to 4.3% THC content by dry weight (Hong and Clarke 1996). Clarke and Merlin (2013) infer that wild forms of asperrima/kafiristanica may have encompassed considerable genetic diversity that included higher THC production. Nevertheless, early cultivars are likely to have been variable and not yet selected for specialized drug or fibre uses. The analysis of a worldwide genomic panel of cannabis by Ren et al. (2021) suggests that these two specialized uses diverged from ancestral general cultivars around the early second millennium bc (ca. 3800 bp). The same study posited domestication as early as the start of the Holocene (ca.12,000 bp; Ren et al. 2021), but the limited sampling of wild populations and wide error bars on such estimates calls for ground truthing such hypothesis through empirical archaeobotanical evidence. For example, the application of similar genetic methods to Asian rice estimated genetic divergence millennia to as much 10,000 years earlier than the first finds of domesticated archaeological remains (Choi et al. 2017). It also should be noted that there are no ancient genomic data to aid calibration of these timescales.
Seed morphology, especially size, varies greatly across the cannabis complex, which offers scope for studying this aspect of evolution through archaeobotanical evidence. Presently more archaeobotanically oriented morphometric work is needed, but the caruncle presence and shape of the hilum/abscission zone do appear to vary between species and sub-species (Fig. 1; e.g. Small and Cronquist 1976; Clarke and Merlin 2013) and suggest that domestication and diversification may be amenable to archaeobotanical analysis. One axis of variation that is currently apparent is that larger seeds are typical of both hemp-fibre and edible oilseed varieties, while seeds of plants cultivated for drugs are generally smaller (Clarke and Merlin 2013, although some modern drug-type seeds can show larger achenes up to ≥ 3.6 mm long, see McPartland and Small 2020: Fig. 3). This pattern of seed size can be compared and contrasted to that of flax, in which early domesticates with larger seeds may have had more importance as oilseeds, while seed size decreased in the Bronze Age with selection for specialized fibre varieties (e.g. Herbig and Maier 2011). In the present contribution we assess existing seed morphometric evidence to assess the antiquity of distinctive fibre /oil seed and drug varieties in eastern Asian cannabis.
Drawings of modern cannabis seeds, adapted from Small and Cronquist 1976. 1 C. sativa ssp. sativa (hemp cultivar), 2 C. sativa ssp. sativa var. spontanea (narrow leaf hemp type ancestor), 3 C. sativa ssp. indica (drug cultivar), 4 C. sativa ssp. indica var. asperrima/kafiristanica (narrow leaf drug type ancestor), scale bar 2 mm. Note the distinctive protruding caruncle in the shattering wild varieties 2 and 4
Cannabis is usually dioecious, having separate male and female plants, apart from plants with monoecious (intersexual) and hermaphroditic (bisexual) flowers. This enforces cross-pollination and maintains diversity within populations. Apart from males not producing seeds, male and female plants also differ by reaching ripeness at different stages, with male plants maturing five to six weeks earlier than females (Edwards and Whittington 1992). This makes the two sexes easily distinguishable and possibly allowed people in the past to select for particular variations, for example to weed out female plants in order to maintain male plants with preferred characteristics (for fibres) or selecting female plants for seeds or psychoactive use by potentially weeding out male plants. However, the dioecious nature of the plant makes it harder to fix selected traits in contrast to self-pollinating species, as are most early seed crops (Clarke and Merlin 2013). In addition to genetic diversity and heterozygosity encouraged by cross-pollination, cannabis is regarded to generally have high phenotypic plasticity (Russo 2007). As explored in Edwards and Whittington (1992) the intended use of cannabis will also impact preferences for the density of male plants maintained in fields. For drug purposes, female plants are preferred, seeds are less needed and so male plants may be fewer (thus less pollen production) given that for some production pollination is generally undesirable as it reduces the phytocannabinoids (Lipson Feder et al. 2021); this is even more so with plants reproduced by cloning. In contrast, for oilseed crops more male plants are needed to ensure all potential seeds are produced through pollination. Similarly, in fibre crops male plants are preferred for producing fibre, which may even be of better quality.
Cannabis grows well on most types of soils, but especially in high nitrogen content soils. The hypothesized original habitat for cannabis (inferred from the optimal growing conditions seen for ruderal cannabis) is a moist, but well drained, open sunny area with a high level of nitrogen in the soil. Growing near streams visited by mammalian herds could have provided the required high levels of nitrogen generated through their urine and dung (Clarke and Merlin 2013). This led to the hypothesis that cannabis was a “camp follower” and may have been first cultivated from volunteer plants on dumps near human habitation (Vavilov 1926; Anderson 1952, p. 167). In nature, cannabis is wind pollinated, however today, whereas cannabis plants bred for fibres and other uses are propagated from seeds or more recently through tissue culture methods (Ranalli 2004; Salentijn et al. 2015; Simiyu et al. 2022), plants bred for medicinal/psychoactive use are mostly cloned through vegetative propagation. Cannabis is particularly invasive of freshly disturbed soil areas (Small 2015), and as a result weedy forms have become widely distributed worldwide, being found on disturbed roadsides, by watercourses, and in cultivated fields. This provides ample scope for introgression that will inevitably complicate historical signals in genetic data.
In recent years, thanks to the increasing deployment of flotation for the recovery of archaeobotanical material during archaeological excavation in Asia, an increasing number of cannabis remains, especially seeds, have been recovered in many early sites across China. Textiles may also be preserved, but accurate identification of bast fibres to species is difficult (e.g. see Catling and Grayson 1982) and rarely reported with convincing details, while claims of identifying hemp textiles from superficial impressions, e.g. on ceramics (McPartland and Hegman 2018; cf. Merlin 2003) are problematic. Pollen has also been widely used to identify the past presence of cannabis. However, wild versus cultivated cannabis cannot be distinguished through pollen. In addition, Cannabis sp. and Humulus sp. (hops) produce morphologically similar pollen grains, which may lead to mistaken identification from the archaeological record (Lewis et al. 1983). The genus Humulus includes widespread, and often weedy, vines native to both Europe (H. lupulus L.) and Asia (H. scandens Lour., syn. H. japonicus Siebold & Zucc., with more localized H. yunnanensis Hu). Cultivated hops, used widely in beer brewing, are cultivars selected from European H. lupulus since the 9th century (Behre 1999). Aside from positively identified textiles of hemp, cannabis seeds from archaeological sites are seen as among the most ubiquitous and reliable indicators of past human use, as opposed to pollen, which indicates local presence of cannabis plants but does not provide information on whether plants were necessarily exploited or cultivated (Long et al. 2017; McPartland et al. 2019; Rull 2022).
Written records on the antiquity and use of Cannabis in China
In early Chinese written texts, cannabis is referred to as má 麻 and most often translated as hemp, implying its use as a fibre plant. The earliest written accounts of cannabis cultivation and use date to the 1st millennium bc (see Table 1, ESM 1 Table S1 for full quotes and translations). It must be noted however that má also became a generic term for a bast fibre or other oil plants, with other kinds of má specified, such as zhùmá 苎麻 for ramie (Boehmeria nivea (L.) Guadich), xúnmá 荨麻 for nettles (Urtica spp.), or zhīmá 芝麻 for sesame (Sesamum indicum L.). Nevertheless, early occurrences of má as cannabis include poems in the Shī Jīng (Book of Odes), where there is a description of how and when to plant cannabis, while descriptions of hemp cloths are recorded in the Shàng Shū (Book of Historical Documents), and the Lǚshì Chūnqiū (Master Lü’s Spring and Autumn Annals). In the Lĭ Jì (Book of Rites), hemp headbands are prescribed to be worn to honor the dead during mourning activities. In the Zhōu Lĭ (the Rites of Zhou), cannabis is grouped with other cereals, including rice, millets, wheat/barley and soybean, attesting to its dual use as fibre and food grain. That cannabis is often described in this and the other works as being cultivated with other cereals, such as millet and wheat, has been interpreted as clear indication of its culinary use. The inferred use of cannabis as food grain is also supported by definitions given in several Běncăo (Chinese traditional Materia Medica), written from the early Eastern Han Dynasty onward in the early first millennium ad (ca. ad 1–200, Brand and Zhao 2017). Within the Běncăo, cannabis is classified as a gŭ, “grain” food crop, together with rice, millets, wheat, and others (Li 2005). The first written evidence relating to a medicinal use of cannabis is found in the Shénnóng Běncăo Jīng, Divine Farmer’s Classic of Materia Medica, traditionally dated to the Western Han Dynasty (first to second centuries ad; Li 1974; Touw 1981). According to this wealth of written evidence, we know that cannabis was known and widely employed in early Chinese societies from at least the first millennium bc, and that the versatile nature of the plant was also understood.
Finally, the first clear written reference to male and female cannabis plants is found in the Ĕr Yă zhù, a commentary by Guō Pú (ad 276–324; see Gao 1996; Clarke and Merlin 2013), based and expanded upon the earlier Ĕr Yă dictionary (itself dated to the Han Dynasty, ca. 206 bc-ad 220; see ESM 1 Table S1).). This commentary refers to cannabis by indicating whether the plant produces seeds, calling it mámù 麻母, or if it does not produce seeds calling it xĭ枲. Later scholars have interpreted xĭ as male cannabis (for hemp production) and mámu as female cannabis (for other uses). This differentiation in male and female plants is seen as indication of an understanding of the dioecious nature of the plant possibly tied with different specific uses (Li 1974; Huang 2000; Clarke and Merlin 2013, p. 203).
Materials and methods
Archaeological and archaeobotanical research at Haimenkou
Haimenkou lies in the Jinsha (Yangtze) river basin at 2,190 m above sea level, in Jianchuan County, northwest Yunnan (26.466914 N, 99.919778 E; Min 2013). This is a mountainous area with distinct dry and wet seasons (between May and October, and November and April, respectively), and an average annual precipitation of 1,000–1,200 ml. After its initial discovery in 1957, Haimenkou underwent several excavation campaigns (YPM 1958; Xiao 1995; YPICRA et al. 2009). The site represents the largest prehistoric site discovered so far in Yunnan, extending over ~ 5 ha (Yao 2010). Large, rectilinear pile dwellings with wooden postholes preserved by waterlogging characterize the site, and the material culture retrieved includes small bronze objects, lithics and bone tools, and ceramic remains (Li and Min 2014). A textile fragment was recovered during the 2008 excavation (Xue et al. 2022: suppl. Material S4F); however no further study or analysis has been carried out so far in order to identify the fibres. Over the course of the 2008 excavation season, archaeobotanical samples for flotation were collected. Laboratory analyses of these samples revealed a flourishing productive economy based on the cultivation of rice and millet for the initial phase of occupation (ca. 1600−1400 bc), followed by the introduction of wheat from ca. 1400 bc, and its increasing importance in the last period of occupation (ca. 800−400 bc, Xue 2010; Xue et al. 2022). Chenopodium (fat hen) was also found in great quantities and associated with cereals remains, especially rice and millet grains, and it has been hypothesized as being cultivated (Dal Martello 2020; Xue et al. 2022). Several fruits and legumes were also found, including soybean (Glycine max), peaches (Prunus persica), apricots (Prunus armeniaca), raspberries (Rubus sp.), grapes (Vitis sp.), melons (Cucumis cf. melo) and jujube (Ziziphus jujuba). Over 800 cannabis grains were recovered in the archaeobotanical samples from Haimenkou. The majority of cannabis seeds (~ 700) were retrieved from a single context dated to 1400−1100 bc (Dal Martello 2020; Xue et al. 2022, Figs. 2 and 3). The cannabis seeds from Haimenkou were preserved by charring and have a slightly elongated shape with a smooth surface, and no pronounced basal caruncle.
Metrics on cannabis achenes and collection of metrics
Thirty grains of cannabis were measured from the archaeobotanical samples of Haimenkou (see ESM 1 Table S2 for measurements on individual grains from Haimenkou); additionally, modern and archaeological measurements of cannabis achenes have been collected from published studies from locations across Eastern and South Asia (China, Japan, Korea and India); further measurements were obtained from published photos (Table 2 lists modern seeds metrics, Table 3 lists available archaeological seeds metrics, see ESM 2 for complete lists and references of both modern and archaeological datasets, see Fig. 4 for location of archaeological sites included in this study). Measurements on modern grains provide guidelines for distinguishing fibre and psychoactive varieties, and modern cannabis metrics have been collected from the available literature. In order to compare modern measurements with archaeological ones, we have applied a correction factor of -10% to account for the shrinkage caused by the charring process, in line with estimated correction factors applied to cereals and pulses when comparing charred vs. non-charred material (Hopf 1955; Hubbard 1976; Willcox 2004; Braadbaart and van Bergen 2005; Fuller and Harvey 2006; Braadbaart 2008; Märkle and Rösch 2008). Most of the archaeological cannabis seeds were preserved by charring; however, cannabis seeds from sites in Xinjiang, including Jiayi, Yanghai, Astana and Karakhoja, were preserved by desiccation; seeds from Torihama in Japan, Shinchangdong in Korea were preserved by waterlogging, and those found in Han Dynasty period graves from the Laoguanshan cemetery in Sichuan were reported as being partially charred. We have applied a correction factor of -10% to the desiccated/waterlogged seeds, in line with shrinkage factors obtained from experimental charring for cereal grains, and of -5% to the partially charred seeds, in order to account for the different preservation status and make the non-charred seeds comparable to charred ones (see Table 3 and ESM 2). We provide both original and corrected measurements in the tables below and in ESM 2, and for the purpose of our analyses, we plot charred and corrected values on the graphs below (Figs. 5, 6 and 7).
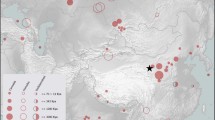
Location of Haimenkou and other sites mentioned in text: 1 Okinoshima; 2 Zhuzhai; 3 Torihama; 4 Yanggua; 5 Hamin Mangha; 6 Jinchankou; 7 Buziping; 8 Kunal; 9 Hetapatti; 10 Erdaojingzi; 11 Shimoyakebe; 12 Gaocheng Taixi; 13 Dazingzhuang; 14 Senuwar; 15 Haimenkou; 16 Guangzhuang; 17 Jiayi; 18 Yanghai; 19 Shinchangdong; 20 Laoguanshan M2; 21 Laoguanshan M3; 22 Marquis Haihun Graveyard; 23 Qara Qorum; 24 Karakhoja; 25 Astana. Made with QGIS

data from Emboden 1974; Small and Cronquist 1976; Russo 2007; Taheri-Garavand et al. 2012; Piluzza et al. 2013; Small 2015; Bouayoun et al. 2018; Asadi et al. 2019; McPartland and Small 2020; Moon et al. 2020; Kaliniewicz et al. 2021
Comparison of modern cannabis achene measurements (shown corrected by -10%, see Table 2 and ESM 2 for original and corrected measurements; see ESM 1 Fig. S1 for indication of provenance;

Archaeological data from Kasahara 1987; Lee 2003; Saraswat and Pokharia 2003; Saraswat 2004; Rösch et al. 2005; Jiang et al. 2006; Chen 2007; Kobayashi et al. 2008; Zhou et al. 2011; Chen et al. 2012; Jia et al. 2013; Sun 2014; Yang 2014; Jiang et al. 2016; Pokharia et al. 2017; Bestel et al. 2018; Chen et al. 2019; Dal Martello 2020; Bai et al. 2021; Jiang et al. 2021
Average seed width of cannabis grains from archaeological sites: 1 Okinoshima; 2 Zhuzhai; 3 Torihama; 4 Yanggua; 5 Hamin Mangha; 6 Jinchankou; 7 Buziping; 8 Kunal; 9 Hetapatti; 10 Erdaojingzi; 11 Shimoyakebe; 12 Gaocheng Taixi; 13 Daxingzhuang; 14 Senuwar; 15 Haimenkou; 16 Guanzhuang; 17 Jiayi; 18 Yanghai; 19 Shinchangdong; 20 Laoguanshan M2; 21 Laoguanshan M3; 22 Marquis Haihun Graveyard; 23 Qara Qorum; 24 Karakhoja; 25 Astana. Charred and corrected values for desiccated, waterlogged and partially charred materials have been plotted (see Table 3 and ESM 2 for original and corrected values).
Results
Modern metrics on cannabis grains
Modern cannabis seeds show distinct sizes for psychoactive and fibre type cannabis (Small 2015, Fig. 5). According to Small and Cronquist’s (1976) early work on cannabis type differentiation, seeds of domesticated cannabis fibre varieties have a length of at least 3.8 mm, with shorter seeds belonging to wild/feral and psychoactive types. Our collection of modern published measurements shows that wild/feral cannabis seeds, which include spontanea and asperrima/kafiristanica varieties, range between 2.24 and 4 mm in length (-10% values: 2.01–3.06 mm), and 1.5–2.8 mm in width (-10% values: 1.35–2.52 mm). Modern psychoactive cannabis seeds range between 3.3 and 4.6 mm in length (-10% values: 3.05–4.1 mm), and 2.4–3.4 mm in width (-10% values: 2.18–3.08). Modern fibre cannabis seeds range between 4.06 and 5.4 mm in length (-10% values: 3.65–4.86 mm) and 2.8−4 mm in width (-10% values: 2.52–3.6 mm), and oil-type cannabis seeds range between 4.2 and 7.1 mm in length (-10% values 3.8–6.4 mm) and between 3.12 and 4.42 mm in width (-10% values 2.8–4.4 mm; Table 2 and ESM 2). Measurements provided in Table 2 comprise modern metrics, both original and corrected by -10% values. Following methods of several recorded crop domestication studies based on grain metrics that showed width is the most affected dimension during the initial domestication phase (e.g. Fuller et al. 2014, 2017, 2019, 2021), we have chosen to plot width for both modern and archaeological seeds in our analyses below; we plot corrected values to allow for comparison with archaeologically charred material. From modern measurements, we consider width ranging between 2.4 and 3.1 mm (-10% corrected value) as the overlapping range between psychoactive and fibre cannabis; width below 2.4 mm (-10% corrected value) as distinctive of psychoactive cannabis, and width above 3.1 mm (-10% corrected value) as distinctive of fibre and oil cannabis. Since fibre and oil type cannabis seed size ranges largely overlap, with all fibre accession falling within the range of oil accessions (Fig. 5), and the two are conventionally recognized as the same subspecies, below we grouped them in the category fibre/oil (Table 2; Figs. 6 and 7).
Haimenkou cannabis grain metrics
The cannabis grains from Haimenkou measured on average 3.39 mm in length, 2.2 mm in width, and 1.2 mm in thickness (see Table 3; Fig. 6; ESM 1 Table S2 and ESM 2). A scatterplot of the measurements from Haimenkou shows that the majority of the seeds plot in the overlapping area between fibre/oil and psychoactive cannabis, according to our collection of modern cannabis seeds metrics. A large number, just over 50%, fall within the expected distribution of wild/feral types, but the absence of a caruncle (Fig. 2) argues against this. While the majority fall within the distribution of psychoactive type, about half fall within the fibre/oil type range with a few grains showing size comparable to only fibre types. We therefore suggest that these represent an early form of ssp. chinensis or ssp. indica before larger seeded oilseed forms had been selected or were available in the region. The archaeological contexts from which cannabis seeds have been retrieved at Haimenkou suggest storage as a food grain, i.e. as oilseed use. This derives from the observation that a single sample, hand collected, contained more than 700 cannabis seeds, thus suggesting charring of a cluster of seeds from part of a stored unit rather than a mixed context. Similar examples of charred samples consisting of almost exclusively clean food grains from the site, included samples with thousands of foxtail millet grains (Setaria italica) and another consisting of rice grains and the pseudo-cereal Chenopodium cf. album (Xue et al. 2022).
Archaeological cannabis grain metrics
Published measurements on archaeological cannabis achenes from sites in East and South Asia have been collected and compared with those obtained from Haimenkou (Table 3; Figs. 5 and 7). Table 3 provides archaeological metrics, including corrected values for desiccated/waterlogged and partially charred archaeological seeds, -10% and -5%, respectively. Average width has been plotted against chronology (median), following methods for tracking grain size change used across many other crops (e.g. Fuller and Allaby 2009; Purugganan and Fuller 2011). This shows that the earliest available reported cannabis grains with metrics (pre-3000 bc) plot within the overlapping area of psychoactive/fibre cannabis. Since even the earliest finds at Okinoshima, Japan (cannabis seeds directly dated) lack a wild-type caruncle, it can be suggested that all of these early records are likely to represent cultivated plants (for definitions of cultivated vs. domesticated plants please see Fuller and Hildebrand 2013). The smaller size is consistent with an early domesticate, but is larger than the expected wild range. Width of grains shows differentiation towards wider grains from ca. 3000 bc onward, suggestive of specialized oilseed varieties. These large seeded examples occur in Xinjiang, represented by the desiccated material found at Yangua, Xinjiang (ca. 3000 bc, Zhou et al. 2011), however Yanghua metrics fall well above modern metrics and might not be totally reliable. Other larger, possible fibre records include Hetapatti (ca. 2000 bc, Pokharia et al. 2017) in India; Gaocheng Taixi (ca. 1600−1046 bc, Chen 2007), and Haimenkou (ca. 1600−400 bc, this study, Xue et al. 2022) in China. It remains to be resolved whether Indian occurrences pre-date 2000 bc (Fuller and Murphy 2018), but this nevertheless implies selection for fibre and oilseed uses began prior than 1500 bc.
A second evolutionary trajectory can be suggested as beginning ca. 2000 bc with a trend towards smaller seeded populations, suggested as including the specialized psychoactive varieties of ssp. indica, perhaps selected for higher THC content, although some feral populations could also be included (Fig. 8). This is represented by finds from the following: Hamin Mangha (ca. 3000 bc, Sun 2014), Erdaojingzi (ca. 2000−1500 bc, Sun 2014) and Yanghai, China (820−300 bc, Jiang et al. 2006); Shimoyakebe, Korea (ca. 3,400 bp, Sasaki et al. 2007; Crawford 2011); Senuwar, India (1400−700 bc, Saraswat 2004). The examples from Yanghai, Shimoyakebe and Senuwar are clearly illustrated and lack a caruncle, indicating these are small-seeded domesticates.
Diagram showing suggested evolution of cannabis, with proposed timeline (approximate in 1,000s of years bp [kya]) of phylum divergence and range expansion events in eastern Eurasia in light of archaeological evidence presented in this paper. For example, post-glacial expansion and radiation takes place between 20,000 and 12,000 bp, domestication episodes take place between 10,000 and 5,000 bp, and special use subspecies are established variously before or after 3,000 bp
Discussion
Although still limited, the available morphometric data shows that all of the archaeological cannabis grain size reports collected for this study are comparable to known grain size in varieties of modern cultivated cannabis. This is consistent with the hypothesis of an early exploitation of the plant. Seeds coming from sites dating from ~ 8000 to ~ 3000 years bc fall in the grey area of undifferentiated cultivars; this would suggest they fit within a broader conception of C. sativa ssp. indica, which plausibly has variable THC content and could indeed have been used for psychoactive purposes (Fig. 8), as argued by Clarke and Merlin (2013). Nevertheless, a generalized use, including some for edible seeds and fibre use is plausible, and food uses are more likely to have resulted in archaeological preservation.
Some of the earliest finds of cannabis in the world come from the site of Okinoshima, Japan; these have been directly dated to around 8000 bc (8280−7660 cal bc, NUTA2-12809, Kobayashi et al. 2008; Kudo et al. 2009). The widths of these seeds fall outside the size range of wild cannabis, and lack the caruncle of var. asperrima/kafiristanica, thus we can infer that these are likely already a domesticated form of ssp. indica. Similarly to Okinoshima seeds, cannabis seeds reported from another Jomon site, Torihama (ca. 5000 bc, Kudo et al. 2009) also, from the photographs, lack a caruncle and no prominent abscission scar is evident (cf. online resource 1 Table S1 in McPartland and Hegman 2018). Whilst the find was interpreted as evidence for introduction and cultivation in Japan (Kobayashi et al. 2008), the early date would suggest that potentially it represents one of the earliest known East Asian domesticates. In China, the earliest reported grains come from Zhuzhai (ca. 5900−5800 bc, Bestel et al. 2018), and their size is greater than known wild seeds, suggesting possible cultivation of cannabis in the Middle Yellow River region of China at an early date. Since there is no evidence for contact between Japan and China at this time for any dispersal of crops (e.g. rice, millets, azuki bean and soybean all only appear to disperse across these regions after 3500 bc, Stevens and Fuller 2017), cultivation of cannabis plausibly had begun independently in at least China and Japan.
Use for fibre and for edible seeds would have been pre-requisites for selection for specialized varieties within ssp. indica var. chinensis, which evolved larger seeds, with the largest found in those varieties specialized for oilseed use. Archaeologically, such large grains have been reported from at least 4000 bc in China (e.g. Yanghua, Gaocheng Taixi), and from perhaps 2000 bc in India (e.g. Hetapatti). This implies that by the 2nd millennium bc, differentiation of hemp for fibre and/or oil seed varieties (ssp. chinensis) from wild varieties had taken place across broader East and South Asia. The selection process for larger seeds is unclear. One possibility is a phase of competitive selection (sensu Allaby et al. 2022), brought about by denser planting or more intensive field preparation, including manuring. Denser planting could also drive selection for taller plants, which came to characterize fibre varieties. It is also possible that larger seeds were brought on by allometric links to larger overall plant size, suggested as playing a role in some domestication processes (Milla and Matesanz 2017).
Early claims of hemp textiles from archaeological sites in China have later been disputed, being mostly based on fabric impressions on ceramics (Bergfjord and Holst 2010; Haugan and Holst 2013). In addition to seeds, securely identified hemp fibre remains come from Gaocheng Taixi, in Hebei, where a complete roll of woven hemp has been recovered (Shang Dynasty, ca. 1600−1046 bc, Cameron 2010). A roll of hemp fabric was also recovered from tomb no. 1 at Mawangdui near Changsha (ca. 200 bc–ad 200, Cheng 1992), and hemp was also reported from Kwo La Wan in Hong Kong (1300−1000 bc, Meacham 1994, pp 184–185). Historical records also show that hemp cloth was used as tax payment during the Zhou dynasty (1045−256 bc), together with grains (Kuhn 1988). At Haimenkou, cannabis seed size mostly plots in the range of overlapping psychoactive/fibre types (Fig. 6); we therefore suggest that the cannabis assemblage from Haimenkou is indicative of a crop beginning to undergo evolution from its early domesticated form towards a diversified crop with multiple uses, including larger oilseed/ fibre adapted varieties. These can probably be attributed to ssp. indica var. chinensis.
In mainland Southeast Asia, at the site of Ban don Ta Phet in central Thailand (ca. 400 bc), hemp has been identified among the numerous textile fragments, and it has been suggested that it was an exotic material coming from China (Cameron 2010). Hemp fibres have also been reported used as clay plaster mixture in the Ellora Cave in India, dating to ca. 6th–11th centuries ad (Singh and Saresdai 2016).
Smaller cannabis seeds appear from ca. 3000 bc, e.g. at Hamin Mangha (China), Shimoyakebe (Japan), and Senuwar (India). While in some cases these might be of wild plants, at least from Jiayi and Yanghai, where seeds have been recovered in association with female plants in burial contexts, suggest it was possibly a medicinal/psychoactive variety (see below).
As seen from the written Chinese records, differentiation of cannabis for fibre/oil use from psychoactive use varieties was coming into existence at least by the first millennium bc. In Indian written sources, early use of the fibre is recorded in Indian languages such as Pali, Prakrit and later Sanskrit, placing it from the first millennium bc through early centuries ad. The Late Vedic Sanskrit (also Pali) śaṇa indicates fibre hemp (Rhys-Davids and Stede 1925; Turner 1966), while Prakrit ganjā is psychoactive hemp (Turner 1966). In addition, bhaṅgá in Pali and later Vedic Sanskrit has glosses for both drug use and fibre use (Rhys Davids and Stede 1925; Turner 1966), which may indicate the persistence of mixed purpose crops. Hymns of the Atharaveda (ca. 1000 bc) also list bhanga alongside other drugs such as soma (Russo 2005). While we have posited psychoactive use since the Early Holocene (as hypothesized by Clarke and Merlin 2013), the higher THC varieties with smaller seeds had evolved by the 2nd millennium bc and 1st millennium bc when they are found in India.
In addition to seeds whole female plants with inflorescence have been reported from graves at the Jiayi and the slightly later Yanghai sites in Xinjiang (Jiang et al. 2006, 2016); these have been interpreted as clear indication of a ritualistic use of the plant (Jiang et al. 2016). At the Han Dynasty period Laoguanshan graves in Sichuan, Southwest China, thousands of seeds have been reported in tombs M3 and M2 (Bai et al. 2021). In tomb M2 in addition to cannabis grains there were four models of weaving machines, suggesting that the deceased buried in M2 was involved in textile production. In Tomb M3 a mortar and pestle and bamboo strips with medical recipes were part of the burial goods, suggesting that the deceased male buried there was a doctor, and further indicating a medicinal use of cannabis by the late 1st millennium bc in China (Bai et al. 2021).
The introduction of cultivated cannabis into India could have possibly derived from Central Asia through the Middle Asian crop exchange (Stevens et al. 2016). The evidence from Yanghai in Xinjiang might suggest that the dispersed variety could have been the drug form. Dispersal into South Asia from Central Asia for cannabis drug cultivars is also inferred on linguistic grounds, as argued on the basis of occurrences of cognate terms in Iranian and Indic languages, implying loans into Indo-Iranian, which Witzel (1999, 2005), Southworth (2005) and Southworth and McAlpin (2014) suggest may have been from a lost Central Asia language: this includes the terms śaṇa and bhaṅgá (Persian šan and bhanga). Witzel (2005) also suggests an original central Asian source *k’an, which would also evolve into ganja, Kirgiz kandir, Old Russian Church Slavic konoplja, and Greek kánnabis. In other words, Middle to Late Bronze Age central Asia would have served as a hub for the diffusion of cannabis varieties, together with their names.
Conclusions
Although limited in scope, this study shows the potential of using grain morphometrics as an aid to disentangle the domestication trajectory and past use of cannabis. Through the collection of modern metrics, we have established a baseline for distinguishing fibre/oil and psychoactive/ritualistic cannabis seeds. This baseline provides a framework within which to analyze archaeological cannabis grains. This, together with a contextual analysis of the archaeological context in which seeds have been found, can provide insights into a more precise, unbiased interpretation of cannabis seeds remains from archaeological sites. According to the presently available data, cannabis exploitation has a long antiquity, with the earliest archaeological seeds already showing sizes comparable to modern varieties, differentiated for fibre or psychoactive use. An initial differentiation between fibre and psychoactive cannabis is detected from ca. 3000 bc for fibre cannabis, and derived oilseed varieties of ssp. chinensis. From ca. 2000 bc, smaller seeded, and presumably high THC psychoactive varieties evolved, and they are present in East Asia and South Asia. Future work should focus on collecting a wider range of measurements.
Data Availability
All data provided is available online as ESM.
References
Allaby RG, Stevens CJ, Fuller DQ (2022) A novel cost framework reveals evidence for competitive selection in the evolution of complex traits during plant domestication. J Theor Biol 537:111004
Anderson E (1952) Plants, Man and Life. University of California Press, Berkeley
APG (The Angiosperm Phylogeny Group) (2003) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc 141:399–436
Asadi S, Moghaddam H, Naghdi Badi H, Naghavi MR, Salami SA (2019) Evaluation of phenotypic properties and seed oil content of hemp (Cannabis sativa L.) ecotypes in Iran. 9th International Conference on Science, Engineering and Technology (WICSET2019). Scientific Society of Paris Universities and WICSET Scientific Society, Paris
Bai Y, Jiang M, Xie T et al (2021) Archaeobotanical evidence of the use of medicinal cannabis in a Secular context unearthed from south China. J Ethnopharmacol 275:114114
Behre K-E (1999) The history of beer additives in Europe—a review. Veget Hist Archaeobot 8:35–48
Bergfjord C, Holst B (2010) A procedure for identifying textile fibers using microscopy: flax, nettle/ramie, hemp and jute. Ultramicroscopy 110:1192–1197
Bestel S, Bao YJ, Zhong H, Chen XC, Liu L (2018) Wild plant use and multi-cropping at the early neolithic Zhuzhai site in the middle Yellow River region, China. Holocene 28:195–207
Biot É (1851) Une –dition r–alis–e par Pierre Palpant, b–n–vole. Le Tscheou-li, Ou rites des tcheou, vol 2. Impr. nationale, Paris
Bouayoun T, Stambouli H, Ez Zoubi Y, El Bouri A, Farah A, Tabyaoui M (2018) Hemp seed oil: Chemical characterization of three non-drug varieties cultivated in Morocco. J Appl Biol Biotechnol 6:37–41
Braadbaart F (2008) Carbonisation and morphological changes in modern dehusked and husked Triticum dicoccum and Triticum aestivum grains. Veget Hist Archaeobot 17:155–166
Braadbaart F, van Bergen PF (2005) Digital imaging analysis of size and shape of wheat and pea upon heating under anoxic conditions as a function of the temperature. Veget Hist Archaeobot 14:67–75
Brand EJ, Zhao ZZ (2017) Cannabis in Chinese Medicine: are some traditional indications referenced in ancient literature related to cannabinoids? Front Pharmacol 8:108. https://doi.org/0.3389/fphar.2017.00108
Cameron J (2010) The Archaeological Textile from Ban Don Ta Phet in broader perspective. In: Bellina B, Bacus EA, Pryce TO, Wisseman Christie J (eds) 50 years of Archaeology in Southeast Asia: essays in Honour of Ian Glover. River Books, London, pp 141–151
Catling D, Grayson J (1982) Identification of Vegetable Fibres. Springer, Dordrecht
Chang K-C (1986) The archaeology of ancient China. Yale University Press, New Haven
Chen X (2007) Paleoethnobotany and agriculture across the transition from the Late Neolithic to the Bronze Age in Northeastern China: A case study. PhD thesis, Shandong University, Jinan (in Chinese)
Chen T, Wu Y, Zhang Y, Wang B, Hu Y, Wang C, Jiang H (2012) Archaeobotanical study of ancient food and cereal remains at the Astana Cemeteries, Xinjiang, China. PLoS ONE 7:e45137
Chen T, He JJ, Yao SW, Qiu ZW, Mai HJ, Wang B, Jiang HG (2019) Scientific analysis of horsetail figurine decoration from the Astana Cemetery in Xinjiang (Xinjiang Astana Gumuqun Chutu Caisu Mawei Jiangshiwu De Kexue Fenxi). Quaternary Sci 39:258–263
Cheng WJ (1992) History of textile technology of ancient China. Science Press, New York
Choi JY, Platts AE, Fuller DQ, Hsing Y-l, Wing RA, Purugganan MD (2017) The rice paradox: multiple origins but single domestication in Asian rice. Mol Biol Evol 34:969–979
Clarke RC, Merlin MD (2013) Cannabis. Evolution and Ethnobotany. University of California Press, Berkeley
Crawford GW (2006) East Asian plant domestication. In: Stark MT (ed) Archaeology of Asia. Blackwell Publishing, Malden, MA, pp 77–95
Crawford GW (2011) Advances in understanding early agriculture in Japan. Curr Anthropol 52(S4):S331–S345
Dal Martello R (2020) Agricultural trajectories in Yunnan, Southwest China: a comparative analysis of archaeobotanical remains from the Neolithic to the Bronze Age. PhD thesis, University College London, London
De Candolle A (1885) Origin of cultivated plants. The International Scientific Series 48. D. Appleton, New York
Edwards KJ, Whittington G (1992) Male and female plant selection in the cultivation of hemp, and variations in fossil Cannabis pollen representation. Holocene 2:85–87
Emboden WA (1974) Cannabis – a polytypic genus. Econ Bot 28:304–310
Fuller DQ, Allaby R (2009) Seed dispersal and crop domestication: shattering, germination and seasonality in evolution under cultivation. Ann Plant Rev 38:238–295
Fuller DQ, Harvey EL (2006) The archaeobotany of Indian pulses: identification, processing and evidence for cultivation. Environ Archaeol 11:219–246
Fuller DQ, Hildebrand E (2013) Domesticating plants in Africa. In: Mitchell P, Lane P (eds) The Oxford handbook of African Archaeology. Oxford University Press, Oxford, pp 506–525
Fuller DQ, Murphy C (2018) Agricultural origins and frontiers in the Indian subcontinent: a current synthesis. In: Korisettar R (ed) Beyond stones and more stones: Domestication of the Indian subcontinent, vol 2. Mythic Society, Bengaluru, India, pp 15–94
Fuller DQ, Denham T, Arroyo-Kalin M et al (2014) Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record. Proc Natl Acad Sci USA 111(6):147–152
Fuller DQ, Colledge S, Murphy C, Stevens CJ (2017) Sizing up cereal variation: patterns in grain evolution revealed in chronological and geographical comparisons. In: Fernández Eraso J, Mujika Alustiza JA, Arrizabalaga Valbuena Á, García Díez M (eds) Miscelánea en homenaje a Lydia Zapata Peña (1965–2015). Servicio Editorial Universidad Del País Vasco, Bilbao, pp 131–149
Fuller DQ, Murphy C, Kingwell-Banham E, Castillo CC, Naik S (2019) Cajanus cajan (L.) Millsp. Origins and domestication: the South and Southeast Asian archaeobotanical evidence. Genet Resour Crop Evol 66:1175–1188
Fuller DQ, Barron A, Champion L, Dupuy C, Commelin D, Raimbault M, Denham T (2021) Transition from wild to domesticated pearl millet (Pennisetum glaucum) revealed in ceramic temper at three Middle Holocene sites in Northern Mali. Afr Archaeol Rev 38:211–230
Gao RG (1996) Guo Pu. In: Feng KZ, Fu QS (ed) Zhuzi baijia da cidian (Dictionary of Philosophers). Liaoning renmin chubanshe, Shenyang, p 86
Haugan E, Holst B (2013) Determining the fibrillar orientation in bast fibres using polarized light microscopy. The modified Herzog test (red plate test) explained. J Microscopy 252:159–168
Henry P, Khatodia S, Kapoor K et al (2020) A single nucleotide polymorphism assay sheds light on the extent and distribution of genetic diversity, population structure and functional basis of key traits in cultivated north American cannabis. bioRxiv. https://doi.org/10.1101/2020.02.16.951459
Herbig C, Maier U (2011) Flax for oil or fibre? Morphometric analysis of flax seeds and new aspects of flax cultivation in late neolithic wetland settlements in Southwest Germany. Veget Hist Archaeobot 20:527–533
Hillig KW (2004) A multivariate analysis of allozyme variation in 93 Cannabis accessions from the VIR germplasm collection. J Ind Hemp 9:5–22
Hillig KW (2005) Genetic evidence for speciation in Cannabis (Cannabaceae). Genet Resour Crop Evol 52:161–180
Hong S, Clarke RC (1996) Taxonomic studies of Cannabis in China. J Int Hemp Assoc 3:55–60
Hopf M (1955) Formveränderung Von Getreidekörnern Beim Verkohlen. Ber Dtsch Bot Ges 68:191–193
Huang HT (2000) Science and Civilisation in China, vol 6: Biology and Biological Technology. Part 5: fermentations and Food Science. Cambridge University Press, Cambridge
Hubbard RNLB (1976) On the strength of the evidence for prehistoric crop processing activities. J Archaeol Sci 3:257–265
Jia X, Dong G, Li H et al (2013) The development of agriculture and its impact on cultural expansion during the late neolithic in the Western Loess Plateau, China. Holocene 23:85–92
Jiang H-E, Li X, Zhao Y-X et al (2006) A new insight into Cannabis sativa (Cannabaceae) utilization from 2500-year-old Yanghai Tombs, Xinjiang, China. J Ethnopharmacol 108:414–422
Jiang H, Wang L, Merlin MD et al (2016) Ancient Cannabis burial shroud in a central eurasian cemetery. Econ Bot 70:213–221
Jiang H, Yang J, Liang T, Zhang Z, Wang S, Qi X, Sheng P (2021) Palaeoethnobotanical analysis of plant remains discovered in the graveyard of the Haihun Marquis, Nanchang, China. Veget Hist Archaeobot 30:119–135
Kaliniewicz Z, Jadwisienczak K, Żuk Z, Lipinski A (2021) Selected physical and mechanical properties of hemp seeds. BioResources 16(1):411–423
Kasahara Y (1987) Torihama Shell Mound. 1985 Survey Summary Report and Research results: survey of low-lying Marsh ruins mainly from the early Jomon Period 6. Wakasa Historical Folk Museum, Fukui Prefecture, pp 1–6. (in Japanese)
Knoblock J, Riegel JK (2000) The annals of Lü Buwei. Stanford University Press, Redwood
Kobayashi M, Momohara A, Okitsu S et al (2008) Fossil hemp fruits in the earliest jomon period from the Okinoshima site, Chiba Prefecture. Jpn J Histor Bot 16:11–18 (in Japanese with English abstract)
Kovalchuk I, Pellino M, Rigault P et al (2020) The genomics of Cannabis and its close relatives. Ann Rev Plant Biol 71:713–739
Kudo Y, Kobayashi M, Momohara A et al (2009) Radiocarbon dating of the fossil hemp fruits in the earliest Jomon period from the Okinoshima Site, Chiba, Japan. Jpn J Histor Bot 17:27–32 (in Japanese with English abstract)
Kuhn D (1988) Textile Technology: spinning and reeling. In: Needham J, Wang L (eds) Science and civilisation in China. Part 9, vol 5. Cambridge University Press, Cambridge, pp 1–520
Lee GA (2003) Changes in subsistence systems in Sothern Korea from the Chulmun to Mumun Periods: Archaeobotanical Investigation. PhD thesis, University of Toronto, Scarborough
Legge J (1876) The Book of Poetry. Create Space Independent Publishing Platform, Scotts Valley
Legge J (1879) The Sacred books of China, part 1 of 6. PART I of the texts of Confucianism. The Shû King, the religious portions of the Shih King, the Hsiâo King. Clarendon Press, Oxford
Legge J (1885) The Sacred books of China, part 3 and 4 of 6. PART III, PART IV of the texts of Confucianism. The Lî Kî. Clarendon Press, Oxford
Lewis WH, Prathiba V, Zenger VE (1983) Airborne and Allergenic Pollen of North America. Johns Hopkins University Press, Baltimore, MD
Li H-L (1974) An archaeological and historical account of cannabis in China. Econ Bot 28:437–448
Li Y (2005) Investigation of the character ma. J Beijing Forestry Univ 4:86–89
Li K, Min R (2014) The site of Haimenkou: new research on the chronology of the early bronze age in Yunnan. In: Hein A (ed) The ‘Crescent-Shaped Cultural-Communication Belt’: Tong Enzheng’s model in Retrospect. An examination of methodological, theoretical and material concerns of long-distance interactions in East Asia: BAR int ser 2679. Archaeopress, Oxford, pp 123–132
Lipson Feder C, Cohen O, Shapira A et al (2021) Fertilization following pollination predominantly decreases phytocannabinoids accumulation and alters the accumulation of terpenoids in Cannabis inflorescences. Front Plant Sci 12:753847
Liu ZX (1999) Further textual research into mafen as recorded in the Divine Farmer’s Materia Medica. J Chin Med Mater 22:211–212
Long T, Wagner M, Demske D, Leipe C, Tarasov PE (2017) Cannabis in Eurasia: origin of human use and bronze age trans-continental connections. Veget Hist Archaeobot 26:245–258
Lu XZ, Clarke RC (1995) The cultivation and use of hemp (Cannabis sativa L.) in ancient China. J Int Hemp Association 2(1):26–30
Märkle T, Rösch M (2008) Experiments on the effects of carbonization on some cultivated plant seeds. Veget Hist Archaeobot 17:257–263
McPartland JM (2018) Cannabis systematics at the levels of family, genus, and species. Cannabis and Cannabinoid Res 3:203–212
McPartland J (2020) Cannabis: the plant, its evolution, and its genetics—with an emphasis on Italy. Rend Lincei Sci Fis Nat 31:939–948
McPartland JM, Hegman W (2018) Cannabis utilization and diffusion patterns in prehistoric Europe: a critical analysis of archaeological evidence. Veget Hist Archaeobot 27:627–634
McPartland JM, Small E (2020) A classification of endangered high-THC cannabis (Cannabis sativa subsp. indica) domesticates and their wild relatives. PhytoKeys 144:81–112
McPartland JM, Hegman W, Long T (2019) Cannabis in Asia: its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies. Veget Hist Archaeobot 28:691–702
Meachan W (1994) On the dating of painted pottery in Hong Kong. Unpubl. paper read at the Conference on Painted Pottery, Chinese University of Hong Kong, February 1994
Merlin MD (2003) Archaeological evidence for the tradition of psychoactive plant use in the old world. Econ Bot 57:295–323
Milla R, Matesanz S (2017) Growing larger with domestication: a matter of physiology, morphology or allocation? Plant Biol 19:475–483
Min R (2013) Haimenkou Yizhi Zonghe Yaniu (Comprehensive study of the Haimenkou site). Xueyuan 15:6–9
Moon Y-H, Cha Y-L, Lee J-E, Kim K-S, Kwon DE, Kang Y-K (2020) Investigation of suitable seed sizes, segregation of ripe seeds, and improved germination rate for the commercial production of hemp sprouts (Cannabis sativa L). J Sci Food Agric 100:2819–2827
Piluzza G, Delogu G, Cabras A, Marceddu S, Bullitta S (2013) Differentiation between fiber and drug types of hemp (Cannabis sativa L.) from a collection of wild and domesticated accessions. Genet Resour Crop Evol 60(2):331–2342
Pokharia AK, Sharma S, Tripathi D, Mishra N, Pal JN, Vinay R, Srivastava A (2017) Neolithic-early historic (2500 – 200 BC) plant use: the archaeobotany of Ganga Plain, India. Quat Int 443:223–237
Purugganan MD, Fuller DQ (2011) Archaeological data reveal slow rates of evolution during plant domestication. Evolution 65:171–183
Qian S, Burton W (1993) (translator) Records of the grand historian: Han dynasty (Vol. 1 & 2). Columbia University Press, New York
Ranalli P (2004) Current status and future scenarios of hemp breeding. Euphytica 140:121–131
Ren M, Tang Z, Wu X, Spengler R, Jiang H, Yang Y, Boivin N (2019) The origins of cannabis Smoking: chemical residue evidence from the first millennium BCE in the pamirs. Sci Adv 5:eaaw1391
Ren G, Zhang X, Li Y et al (2021) Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa. Sci Adv 7:eabg2286
Rhys Davids TW, Stede W (1925) The Pali text Society’s Pali–English dictionary. Pali Text Society, Chipstead
Rösch M, Fischer E, Märkle T (2005) Human diet and land use in the time of the khans—archaeobotanical research in the capital of the Mongolian Empire, Qara Qorum, Mongolia. Veget Hist Archaeobot 14:485–492
Rull V (2022) Origin, early expansion, domestication and anthropogenic diffusion of Cannabis, with emphasis on Europe and the Iberian Peninsula. Perspect Plant Ecol Evol Syst 55:125670
Russo E (2005) Cannabis in India: ancient lore and modern medicine. In: Mechoulam R (ed) Cannabinoids as therapeutics. Milestones in Drug Therapy MDT. Birkhäuser, Basel, pp 1–22. https://doi.org/10.1007/3-7643-7358-X
Russo EB (2007) History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers 4:1614–1648
Salentijn EM, Zhang Q, Amaducci S, Yang M, Trindade LM (2015) New developments in fiber hemp (Cannabis sativa L.) breeding. Ind Crops Prod 68:32–41
Saraswat KS (2004) Plant economy of early farming communities at Senuwar, Bihar. In: Singh BP (ed) Early farming communities of the Kaimur: excavations at Senuwar. Publication Scheme, Jaipur, pp 416–535
Saraswat KS, Pokharia AK (2003) Palaeoethnobotanical investigations at early Harappan Kunal. Pragdhara 13:105–140
Sasaki Y, Kudo Y, Momohara A (2007) Utilization of plant resources reconstructed from plant macrofossils during the latter half of the Jomon period at the Shimo-Yakebe site, Tokyo. Jpn J Histor Bot 15:35–50
Sawler J, Stout JM, Gardner KM et al (2015) The genetic structure of marijuana and hemp. PLoS ONE 10:e0133292
Simiyu DC, Jang JH, Lee OR (2022) Understanding Cannabis sativa L.: current status of propagation, Use, legalization, and haploid-inducer-mediated genetic Engineering. Plants 11:1236
Simpson MG (2010) Plant Systematics, 2nd edn. Academic Press, New York
Singh M, Sardesai MM (2016) Cannabis sativa (Cannabaceae) in ancient clay plaster of Ellora Caves, India. Curr Sci 110:884–891
Small E (1975) Morphological variation of achenes of Cannabis. Can J Bot 53:978–987
Small E (2015) Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. Bot Rev 81:189–294
Small E (2017) Cannabis. A complete guide. CRC Press, Boca Raton
Small E, Cronquist A (1976) A practical and natural taxonomy for Cannabis. Taxon 25:405–435
Southworth FC (2005) Linguistic archaeology of South Asia. Abingdon. Routledge, New York
Southworth FC, McAlpin D (2014) South Asia: dravidian linguistic history. The Global Prehistory of Human Migration. Wiley Blackwell, Chicester, West Sussex
Stevens CJ, Fuller DQ (2017) The spread of agriculture in eastern Asia: Archaeological bases for hypothetical farmer/language dispersals. Lang Dyn Chang 7:152–186
Stevens CJ, Murphy C, Roberts R, Lucas L, Silva F, Fuller DQ (2016) Between China and South Asia: a middle Asian corridor of crop dispersal and agricultural innovation in the bronze age. Holocene 26(1):541–555
Sun Y (2014) Research on plant remains from Neolithic to Early Bronze Age in Upper West Liao River region (Xi Liaohe Shangyou Diqu Xinshiqi Shidai Zhi Zaoqi Qintong Shidai Zhiwu Yicun Yanjiu). PhD thesis, Inner Mongolia Normal University, Hohhot
Taheri-Garavand A, Nassiri A, Gharibzahedi SMT (2012) Physical and mechanical properties of hemp seed. Int Agrophys 26:211–215
Tang L, Zheng Y, Zhu J, Tian J, Gao X, Han G (2022) Study on the utilization of crops in Zhou Dynasty in the Zhengzhou region: a case from Guanzhuang site. Xingyang. Quat Sci 42(1):129–143
Touw M (1981) The religious and medicinal uses of Cannabis in China, India, and Tibet. J Psychoact Drugs 13:23–34
Turland NJ, Wiersema JH, Barrie FR et al (2018) International Code of nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Koeltz Botanical Books, Oberreifenberg
Turner RL (1966) A comparative dictionary of the Indo-aryan languages. Oxford University Press, London
Vavilov NI (1926) Studies on the origin of cultivated plants. Institute of Applied Botany and Plant Improvement, Leningrad
Vavilov NI, Dorofeyev VF (1992) Origin and geography of cultivated plants. Cambridge University Press, Cambridge
Willcox G (2004) Measuring grain size and identifying Near Eastern cereal domestication: evidence from the Euphrates valley. J Archaeol Sci 31:145–150
Witzel M (1999) Early Sources for South Asian Substrate Languages. Mother Tongue (Special Issue) 1999:1–70
Witzel M (2005) Central Asia roots and acculturation in South Asia: linguistic and archaeological evidence from western Central Asia, the Hindukush and Northwestern South Asia for Early Indo-Aryan Language and Religion. In: Osada T (ed) Linguistics, Archaeology and the human past. Indus Project, Research Institute for Humanity and Nature, Kyoto, pp 87–211
Wu ZY, Raven PH, Hong DY (2003) Flora of China, Vol 5: Ulmaceae through Basellaceae. Missouri Botanical Garden Press, St. Louis. http://www.efloras.org. Accessed 16 May 2022
Xiao MH (1995) The early bronze age site of Haimenkou, Jianchuan, Yunnan (Yunnan Jianchuan Haimenkou Qingtong Shidai Zaoqi Yizhi). Kaogu 9:775–787
Xue Y (2010) Preliminary Investigation on the Archaeobotanical Material from the Site of Haimenkou in Jianchuan County, Yunnan (Yunnan Jianchuan Haimenkou Yizhi Zhiwu Yicun Chubu Yanjiu). MA thesis, Department of Archaeology and Museology, Peking University, Beijing
Xue Y, Dal Martello R, Qin L, Stevens CJ, Min R, Fuller DQ (2022) Post-neolithic broadening of agriculture in Yunnan, China: archaeobotanical evidence from Haimenkou. Archaeol Res Asia 30:100364
Yang S (1998) The Divine Farmer’s Materia Medica: a translation of the Shen Nong Ben Cao Jing. Blue Poppy Enterprises, Inc, Boulder, CO
Yang Y (2014) The analysis of charred plant seeds at Jinchankou site and Lijiaping site during Qijia Culture period in the Hehuang region, China. Master thesis, Lanzhou University, Lanzhou
Yao A (2010) Recent developments in the archaeology of southwestern China. J Archaeol Res 18:203–239
YPICRA (Yunnan Provincial Institute of Cultural Relics and Archaeology) et al et al (2009) The third excavation on the Haimankou site in Jianchuan, Yunnan (Yunnan Haimenkou Yizhi Di san Ci fajue). Kaogu 8:3–22
YPM (Yunnan Provincial Museum) (1958) Jianchuan Haimenkou Gu Wenhua Yizhi Qingli Jianbao (preliminary report on excavations conducted at the ancient cultural site of Haimenkou, Jianchuan). Kaogu Tongxun 6:5–12
Zhang Q, Chen X, Guo H et al (2018) Latitudinal adaptation and genetic insights into the origins of Cannabis sativa L. Front Plant Sci 9:1876
Zhou X, Li X, Zhao K, Dodson J, Sun N, Yang Q (2011) Early agricultural development and environmental effects in the neolithic Longdong basin (eastern Gansu). Chin Sci Bull 56:762–771
Acknowledgements
We are grateful to Yuchao Jiang and Yu Gao for help with flotation and aspects of field work at the 2008 excavation of Haimenkou. Excavation, hand-collection sampling and flotation work led by MR at the site of Haimenkou was supported by the National Administration of Cultural Heritage, China. RDM’s doctorate research was funded by the Arts and Humanities Research Council (AHRC) London Arts and Humanities Partnership (LAHP) doctoral studentship. Aspects of laboratory analysis were supported by the European Research Council (ERC) advanced grant “Comparative Pathways to Agriculture” (no. 323842). Additional support was provided by the ERC starting grant “Fruits of Eurasia, Domestication and Dispersal” (no. 851102) awarded to Dr. Rob Spengler, and by the European Union—Next Generation EU—Project PE00000020—CHANGES—Cultural Heritage Active Innovation for Sustainable Society, CUP H53C22000850006.
Author information
Authors and Affiliations
Contributions
Conceptualization: RDM and DQF; methodology: RDM and DQF; formal analysis: RDM, CJS and DQF; data collection and curation: RDM, CJS and DQF; writing—original draft preparation: RDM; writing—Introduction original draft preparation DQF; writing—review and editing: RDM, RM, LQ, CJS and DQF; visualization: RDM, CJS and DQF. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by F. Bittmann.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dal Martello, R., Min, R., Stevens, C.J. et al. Morphometric approaches to Cannabis evolution and differentiation from archaeological sites: interpreting the archaeobotanical evidence from bronze age Haimenkou, Yunnan. Veget Hist Archaeobot (2023). https://doi.org/10.1007/s00334-023-00966-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00334-023-00966-6