Abstract
Objective
To analyze changes in the muscular fat fraction (FF) during immobilization at the intensive care unit (ICU) using dual-energy CT (DECT) and evaluate the predictive value of the DECT FF as a new imaging biomarker for morbidity and survival.
Methods
Immobilized ICU patients (n = 81, 43.2% female, 60.3 ± 12.7 years) were included, who received two dual-source DECT scans (CT1, CT2) within a minimum interval of 10 days between 11/2019 and 09/2022. The DECT FF was quantified for the posterior paraspinal muscle by two radiologists using material decomposition. The skeletal muscle index (SMI), muscle radiodensity attenuation (MRA), subcutaneous-/ visceral adipose tissue area (SAT, VAT), and waist circumference (WC) were assessed. Reasons for ICU admission, clinical scoring systems, therapeutic regimes, and in-hospital mortality were noted. Linear mixed models, Cox regression, and intraclass correlation coefficients were employed.
Results
Between CT1 and CT2 (median 21 days), the DECT FF increased (from 20.9% ± 12.0 to 27.0% ± 12.0, p = 0.001). The SMI decreased (35.7 cm2/m2 ± 8.8 to 31.1 cm2/m2 ± 7.6, p < 0.001) as did the MRA (29 HU ± 10 to 26 HU ± 11, p = 0.009). WC, SAT, and VAT did not change. In-hospital mortality was 61.5%. In multivariable analyses, only the change in DECT FF was associated with in-hospital mortality (hazard ratio (HR) 9.20 [1.78–47.71], p = 0.008), renal replacement therapy (HR 48.67 [9.18–258.09], p < 0.001), and tracheotomy at ICU (HR 37.22 [5.66–245.02], p < 0.001). Inter-observer reproducibility of DECT FF measurements was excellent (CT1: 0.98 [0.97; 0.99], CT2: 0.99 [0.96–0.99]).
Conclusion
The DECT FF appears to be suitable for detecting increasing myosteatosis. It seems to have predictive value as a new imaging biomarker for ICU patients.
Clinical relevance statement
The dual-energy CT muscular fat fraction appears to be a robust imaging biomarker to detect and monitor myosteatosis. It has potential for prognosticating, risk stratifying, and thereby guiding therapeutic nutritional regimes and physiotherapy in critically ill patients.
Key Points
-
The dual-energy CT muscular fat fraction detects increasing myosteatosis caused by immobilization.
-
Change in dual-energy CT muscular fat fraction was a predictor of in-hospital morbidity and mortality.
-
Dual-energy CT muscular fat fraction had a predictive value superior to established CT body composition parameters.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Critically ill patients in the intensive care unit (ICU) commonly suffer from sarcopenia [1]. Sarcopenia is defined as low muscle strength with loss of muscle mass or loss of muscle quality [2]. It is primarily caused by a high age (primary sarcopenia). Secondary sarcopenia can be caused by risk factors, such as malnutrition, inflammation, cardiopulmonary complications, and inactivity, all of which are frequent in critically ill patients [3]. Sarcopenia results in adverse health outcomes, such as falls, fractures, metabolic syndrome, depression, postoperative complications, and poor overall survival [4]. If detected at an early stage, irreversible muscle wasting can be prevented by regular physical activity, neuromuscular electrical stimulation in immobilized patients, and nutritional support [5]. The effective treatment of sarcopenia leads to a reduced length of ICU- and hospital stay, as well as decreased rates of delirium and sedation in the critically ill [6, 7].
Low muscle strength is indicative of sarcopenia [1]. The diagnosis should then be confirmed by measuring reduced muscle mass or muscle quality [1]. Muscle strength is usually clinically assessed, e.g., by measuring the hand grip strength [1]. However, this requires awake and cooperative patients and is thus challenging in the ICU [7]. Similarly, the quantification of both muscle quantity and quality is frequently hindered in critically ill patients. Muscle mass can be quantified using bioelectrical impedance analysis (BIA), ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI). However, BIA and ultrasonography are influenced by muscular edema, operator-dependent, and of disputed diagnostic accuracy [8]. Even if not the most common in clinical routine, MRI and CT are thus considered the gold standard [9]. In ICU patients, CT is the most convenient imaging modality, as these patients often require repetitive CT examinations for clinical reasons. Also, the use of MRI is frequently limited due to in-part ferromagnetic medical equipment in ICU patients. With CT, the skeletal muscle mass can be assessed based on the appendicular, psoas, or whole abdominal muscle area [2].
Muscle quality is defined as micro- and macroscopic aspects of muscle architecture and composition [1], which explain muscle strength per unit size of muscle mass. It is dependent on the deposition of fat inside the skeletal muscle [10], the so-called myosteatosis [10]. Myosteatosis is closely linked to sarcopenia [11], despite being recognized as a distinctive disease [12]. Myosteatosis is caused by various mechanisms, such as the adipogenic conversion of precursor stem cells due to increased glucocorticoid levels [12, 13]. The prognostic relevance of myosteatosis has been shown in various patient groups, such as in different cancer entities [14]. Myosteatosis commonly serves as a parameter of muscle quality for body composition analyses in CT and MRI [15]. To assess myosteatosis with MRI, it is necessary to prospectively select suited sequences for fat quantification. In CT, only indirect and easily biased options existed to determine myosteatosis. Besides subjective grading of muscle morphology [16], most commonly, the CT density, the so-called muscle radiodensity attenuation (MRA), is applied to assess myosteatosis and, thus, muscle quality. Even if the MRA has a predictive value for many entities [12, 17], its use is limited due to the impact of contrast agent on muscle density.
Recently, it has thus been proposed to use dual-energy CT (DECT) material quantification to directly quantify the muscular fat content (FF) unbiased by contrast agent [18]. Good agreement between the DECT FF and MRI chemical shift relaxometry was demonstrated [18], as well as the ability to distinguish between different MR grades of fat infiltration [19]. However, the applicability of the DECT FF to monitor myosteatosis in a clinical cohort and its potential predictive value were not investigated, yet.
Hence, this study aimed to investigate changes in the muscle status in immobilized ICU patients using DECT FF and conventional CT parameters of muscle quantity (skeletal muscle index (SMI)) and quality (MRA). Secondly, we evaluated the predictive value of conventional CT parameters and the DECT FF as a new imaging biomarker for morbidity and survival.
Methods
This retrospective observational study was approved by the local ethics committee (Ärztekammer Hamburg, PV7006-4406-BO-ff). All analyses were conducted in accordance with the Declaration of Helsinki and in compliance with local ethical guidelines. The article is reported in accordance with the STROBE guidelines [20].
Study population
Included were ICU patients who a) were intubated and thus immobilized, and b) received two contrast-enhanced abdominal DECT scans within a minimum time interval of ten days between November 2019 and September 2022. The minimum time interval of ten days was chosen, as this study aimed to investigate the suitability of the DECT FF to detect changes in muscle status in comparison to other CT body composition parameters. Thus, a sufficiently long period of immobilization was necessary for changes in muscle status to occur. According to the literature, muscle changes can occur very quickly, but have likely occurred within 14 days (with variations depending on the collective and the muscle group) [21]. Exclusion criteria were a) age under 18 years, b) discharge from the ICU between CT scans, and c) artifacts from metal implants that reduced CT image quality. Other parameters that may impact image quality, such as ascites or anasarca were noted, but patients were not excluded, as the DECT FF was nevertheless expected to be applicable.
DECT image acquisition
All patients received DECT scans using a dual-source CT scanner (SOMATOM Force, Siemens Healthineers, Erlangen, Germany). Scan parameters were 100 kV and 150 kV with a tin filter, pitch 0.5, collimation 0.6 mm, slice thickness 1 mm (reconstructed slice thickness: 5 mm), pixel size 0.6 × 1 mm. Images were acquired 80 seconds after injection of 80 mL Iomeprol contrast agent (Imeron 350 M, Bracco IMAGING, Milan, Italy).
DECT muscle fat quantification
The DECT FF was quantified using the standard software of the CT scanner’s manufacturer (syngo.via, Siemens Healthineers, Erlangen, Germany). Details of the postprocessing have been previously described in detail and validated by Molwitz et al [18]. To summarize, virtual noncontrast images were created, based on three material decomposition for soft tissue, iodine, and fat using the syngo.via “Liver VNC” tool. With the “Liver fat map” tool, quantified fat values and a color-coded fat concentration map can be displayed as an overlay on the CT grey-scale images. Regions of interest (ROI) (median 8.1 cm2) were drawn on transverse CT images with fat map overlays by contouring the inner circumference of the posterior paraspinal muscle on both sides of the spine at the height of the third lumbar vertebra (L3). The height of L3 was chosen as the muscle area at this height has been demonstrated to correlate best with the whole-body muscle mass [22]. It is thus the standard measurement height for body composition analyses and allows direct comparison to other studies. ROIs were defined for each patient on three adjacent slices to enhance the robustness of the measurement results. The fat fraction (%) was noted for each ROI and averaged per side and per patient. All DECT analyses were performed independently by two radiologists (4 and 2 years of experience).
Assessment of conventional CT body composition parameters
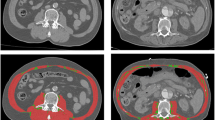
For each patient, a transverse CT image at the mid-height of L3 was exported from the radiological Picture Archiving and Communication system (PACS, GE Centricity, Milwaukee, USA). For further processing, the open-source software Image J (National Institutes of Health) was used in line with a step-by-step guide previously published by Gomez-Perez et al [23]. Figure 1 shows these post-processing steps in an exemplary study patient. First, the circumference of the inner and outer abdominal and paraspinal musculature, as well as the circumference of L3 was outlined. After application of a muscle-specific threshold (−29 to +150 Hounsfield units [HU]), the area within the inner perimeter of the musculature (Fig. 1B) and of the vertebra (Fig. 1C) were subtracted from the area within the outer perimeter of the musculature (Fig. 1A). The derived skeletal muscle area was divided by square body height (m) to provide the SMI (cm2/m2). The MRA (HU) was given by the mean density of the whole abdominal muscle area (Fig. 1F). The waist circumference (WC, cm) was assessed by contouring the body circumference at the height of L3. The subcutaneous adipose tissue area (SAT, cm2) was calculated by subtracting the area within the outer muscle circumference (Fig. 1D) from the whole-body area after application of a fat-specific threshold (−150 to −30 HU). The visceral adipose tissue area (VAT, cm2) was derived from the area within the inner muscle perimeter (Fig. 1E) using the same fat-specific threshold.
Postprocessing contours to quantify conventional CT body composition parameters. Displayed is the outer (A) and inner (B) perimeter of the abdominal and paraspinal muscle, and of the third lumbar vertebra (C), as well as the whole abdominal muscle area (F) each with the muscle-specific threshold of –29 to +150 HU. With a fat-specific threshold of −150 to −30 HU, (D) displays the outer and (E) the inner muscle perimeter. The skeletal muscle index is then given according to Gomez-Perez et al [23] by subtracting B and C from A. The muscle radiodensity attenuation is given by F. The subcutaneous adipose tissue is given by the area outside the defined muscle perimeter in D, and the visceral fat by the area within the perimeter in E
Assessment of clinical parameters
Dates of hospital and ICU admission/dismissal, or death were noted. Reasons for ICU admission were determined and categorized in post-surgery vs. other medical indications. The “Simplified Acute Physiology Score II” (SAPS II), “Sequential Organ Failure Assessment” (SOFA), and the “Charlson Comorbidity Index” (CCI) were assessed at ICU admission. Patient diseases that were assessed as part of the CCI were clustered into the following categories: known malignancies (solid tumor, leukemia, or lymphoma), chronic diseases (chronic obstructive pulmonary disease; chronic heart failure defined as exertional or paroxysmal nocturnal dyspnea which responded to digitalis, diuretics, or afterload-reducing agents; liver failure which is categorized into mild (no portal hypertension), moderate (with portal hypertension), and severe (with portal hypertension and variceal bleeding history); renal disease defined as moderate (creatinine > 3 mg/dL) or severe (on dialysis or status post kidney transplant)). Laboratory measurements (platelets, lactate, bilirubin, creatinine) were noted at the time of ICU admission. Also, data were collected on therapeutic measurements (invasive ventilation, tracheotomy, renal replacement therapy, use of vasopressors, and parenteral nutrition).
Statistical analysis
Continuous data are represented using mean with standard deviation if normally distributed or median with range (min-max) and categorical data via absolute and relative frequencies. The inter-observer variability was determined using intraclass correlation coefficients (two-way mixed, absolute agreement) (ICC). Due to the different units of measurement, the change in DECT FF, SMI, and MRA from CT1 to CT2 was standardized using a z-transformation. Further, the measurement difference was then divided by the individual time difference between CT1 and CT2 for each patient. The values of the MRA and SMI (where a decrease was expected) were multiplied with –1 to make them comparable to the DECT FF measurements. Spearman correlation coefficients (continuous variables) or point biserial correlation (categorical with continuous variables) were assessed. Linear mixed models were employed, including the CT parameters at CT1, the change in CT parameters standardized to the varying time intervals between CT1 and CT2, and the following independent variables: age, sex, body mass index (BMI), the reason for ICU stay (surgical vs. non-surgical), and diseases (malignancies, chronic diseases, chronic inflammatory diseases, renal diseases). The random effect was the patient. Uni- and multivariable Cox-regression analyses were used to test the association between the changes in body composition parameters standardized to the varying time intervals between CT1 and CT2 with in-hospital mortality, new renal replacement therapy (including sex, age, BMI, SAPS II, SOFA, CCI, creatinine at admission, and known renal disease) and tracheotomy at ICU (including sex, age, BMI, SAPS II, SOFA, and CCI). The clinical scoring systems (SAPS II, SOFA, and CCI) were included as independent variables instead of outcome variables as infections or sepsis (SAPS II, SOFA), or comorbidities (CCI) were already commonly present at ICU admission. Model assumptions were checked graphically via histograms of residuals for linear models and Schoenfeld residuals, as well as martingale residuals for the Cox model. There were no missing data. No adjustment for multiple testing was conducted due to the explorative design of the study. Because of the explorative design, p values are descriptive. Statistical analyses were conducted using R version 4.2.3 and SPSS (Version 28.0.1.1, IBM, Armonk, NY).
Results
Study Population
A total of 81 patients were included in the final study population (35 (43.2%) female, average age 60.3 years ± 12.7). The median time difference between CT1 and CT2 was 21 days (range: 10–195). The median time difference from ICU admission to CT1 was 8 days (range: 0–175). At ICU admission (CT 1), ascites or anasarca were present in 56 patients (68%). Patients spent a median time of 53 days (range: 13–321) in the ICU. Of all patients, 48 (61.5%) died during the hospital stay, and 45 died in the ICU. Table 1 illustrates patient characteristics as well as laboratory values at ICU admission.
Body composition results of CT1
On average, male patients showed a −6.7% lower DECT FF than female patients [95% confidence interval (CI) −12.2; −1.2], p = 0.018). The SMI was higher in male patients (4.5 cm2/m2 [0.7; 8.2], p = 0.020), as was the VAT (68.4 cm2/m2 [34.4; 102.4], p < 0.001). A higher age was associated with a higher DECT FF (0.3% [0.1; 0.5], p = 0.014) and VAT (1.6 cm2 [0.3; 2.9], p = 0.021). A higher BMI was associated with increased WC, SAT, and VAT (all p < 0.001). SMI, MRA, and DECT FF were not influenced by BMI. SMI at CT1 was lower if patients received renal replacement therapy (−8.2 cm2/m2 [−13.7; −2.7], p = 0.004). Table 2 shows the effects of different patient characteristics on measurement values of the DECT FF, MRA, and SMI at CT1, Supplementary Table 1 shows the effect of these variables on the WC, SAT, and VAT.
Change in body composition between CT1 and CT2
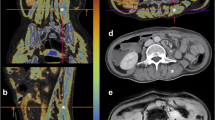
The effect of independent variables (e.g., sex, comorbidities) on the change of CT body composition parameters standardized to the varying time intervals between CT1 and CT2 is shown in Table 3 and Supplement, Table 2. The changes in CT parameters over time are schematically illustrated in Fig. 2. The DECT FF increased from 20.9% ± 12.0 to 27.0% ± 12.0 (effect size: 0.08 [0.03; 0.12], p = 0.001), while the MRA decreased from 29 HU ± 10 to 26 HU ± 11 with a proportionally smaller effect size (effect size: 0.05 [−0.08; −0.01], p = 0.009). The SMI also decreased from 35.7 ± 8.8 to 31.1 ± 7.6 cm2/m2 (effect size: −0.09 [−0.12; −0.05], p < 0.001). WC, SAT, and VAT did not change significantly. Figure 3 portrays the DECT FF and MRA at CT1 and CT2 in an exemplary 56-year-old female patient with severe pneumonia. The time interval between CT1 and CT2 in this patient was 22 days. The DECT FF increased from 3.6% at CT1 to 19.7% at CT2, while the MRA decreased from 37 to 35 HU.
Change of CT parameters during immobilization at the intensive care unit. The skeletal muscle index (SMI) as a measure of muscle mass decreased over time. The DECT FF increased and the muscle radiodensity attenuation (MRA) in HU decreased, both demonstrating progressive myosteatosis. Decreases of waist circumference (WC), subcutaneous and visceral adipose tissue area (SAT and VAT) were less pronounced with large standard deviations
Changes of DECT FF (A) and MRA (B) during immobilization of a 56-year-old female patient with pneumonia. This patient received mechanical ventilation, parenteral nutrition, and renal replacement therapy at intensive care unit, and died 3 days after CT2. The time interval between CT1 and CT2 was 22 days. A marked increase in the DECT FF and thus an increase of myosteatosis was observed. The change of the muscle radiodensity attenuation (MRA) was less pronounced, potentially due to the impact of the contrast agent on the MRA. The skeletal muscle index (not depicted) also decreased from 34.5 to 28.1 cm2/m2
Correlation between CT body composition parameters
The DECT FF showed a good correlation with the MRA (r = −0.75 at CT1; r = −0.72 at CT2) and almost no correlation with the SMI (r = −0.18 at CT1; r = −0.21 at CT2). The increase in DECT FF also correlated well with the decrease in MRA (r = 0.61), while the correlation with the decrease of the SMI was weak (r = 0.23).
Association between CT parameters and morbidity/mortality
The relationships between the changes of the DECT FF, MRA, and SMI per day, standardized to the varying time intervals between CT1 and CT2, with in-hospital mortality after CT2 are shown in Table 4 and Fig. 4. In the multivariable analyses, the increase of the DECT FF per day was associated with in-hospital mortality (hazard ratio (HR): 9.20 [1.78; 47.71], p = 0.008), while the changes of the MRA and SMI were not. Of all other investigated parameters, only a higher age (HR: 1.03 [1.00; 1.07], p = 0.037) and male sex (HR: 2.52 [1.17; 5.45], p = 0.019) were associated with mortality (Fig. 4). In the univariable analysis, both, changes of the DECT FF and the MRA were significantly associated with mortality, but the increase in DECT FF showed the highest HR. The absolute measurement values of the DECT FF, MRA, or SMI at CT1 or CT2 were not associated with in-hospital mortality.
Association between the clinical and CT parameters with in-hospital mortality. The change of the DECT FF per day was associated with in-hospital mortality, while neither the changes of the skeletal muscle index (SMI), of the muscle radiodensity attenuation (MRA) nor the clinical parameters and scores were
The relationships between the changes of the DECT FF, MRA, and SMI per day standardized to the varying time intervals between CT1 and CT2 and the incidence of a new renal replacement therapy or tracheotomy at ICU are shown in Table 5. In the univariable analysis, the relative increase of the DECT FF showed the strongest association with new renal replacement therapy and tracheotomy at the ICU. In the multivariable models, only the relative increase of the DECT FF per day was associated with the incidence of renal replacement therapy (HR 48.67 [9.18; 258.09], p < 0.001) and tracheotomy (HR 37.22 [5.66; 245.02], p < 0.001) (Table 5).
Inter-observer reproducibility
The inter-observer reproducibility of DECT FF measurements was almost perfect (ICC: 0.98 [0.97; 0.99] at CT1 and 0.99 [0.96; 0.99] at CT2).
Discussion
While the DECT FF had been used for fat quantification in the liver, bone marrow, and adrenal glands [24], it has only recently been proposed as a new biomarker of myosteatosis [18, 25, 26]. This retrospective observational study was the first to investigate changes in the muscle status with DECT FF and to evaluate its predictive value as a new imaging biomarker compared to conventional CT body composition parameters. The major findings of this study were that a) myosteatosis, measured by the DECT FF and MRA, increased during immobilization in the ICU while the SMI and thus muscle mass decreased and b) the increase of the DECT FF was proportionally greater than the decrease of the MRA. Also, c) only the standardized increase in DECT FF was associated with the incidence of new renal replacement therapy and tracheotomy at ICU, as well as with in-hospital mortality, while conventional CT parameters were not.
During immobilization at the ICU, a degradation of muscle quantity and quality (as indicated by muscular fat content [27]) was expected. While the decrease in muscle mass (SMI) is often investigated in critical illness [28, 29], myosteatosis is a poorly explored imaging aspect of muscle weakness in critically ill patients. In this study, the muscle mass (SMI) and muscle density (MRA) decreased from CT1 to CT2, while the DECT FF increased. Previous studies similarly reported a decrease in SMI and MRA over time [30, 31]. However, this was the first time that the DECT FF was applied as a biomarker of myosteatosis in critically ill patients. Interestingly, the changes of the DECT FF and MRA were independent of the BMI. Although myosteatosis is often described in the context of obesity [32], it has been found to develop independent of body weight before [33]. In previous studies, fatty muscle infiltration was also independent of muscle mass [11, 34] and preceded muscle atrophy [32]. That would explain the low correlation between DECT FF and SMI in this study.
Although the decrease of the MRA and increase of the DECT FF both indicated progressive myosteatosis, the increase in DECT FF was more pronounced. This is likely, because opposed to the DECT FF, the MRA is influenced by iodinated contrast agent and the contrast timing of the CT scan [35]. Thus, analyses of the MRA are less reliable. Moreover, in many studies, information on the use of contrast agent or scan phase is missing, which hinders study comparability regarding the MRA, as found by a review of Poltronieri et al of 117 studies [15]. In this context, DECT material decomposition is beneficial, because it inherently distinguishes between materials with different atomic numbers, such as iodine and fat [36]. Hence, the quantity of contrast agent in the respective tissue does not bias fat quantification results.
Regarding the clinical patient outcome, in multivariable analyses only the change in DECT FF per day standardized to the varying time interval between CT1 and CT2 was associated with new renal replacement therapy and tracheotomy at the ICU, as well as in-hospital mortality. These results are contradictory to the previous literature, which reported a relationship between the MRA and mortality in critically ill patients [37, 38]. They may be explained by the inherent limitations of the MRA as discussed above. Still, these results indicate that myosteatosis could have a greater association with morbidity and mortality than muscle quantity. This phenomenon has been described before, e.g., in a meta-analysis including patients with COVID-19 [39], in cancer patients [40, 41], or patients with liver surgery [42, 43]. Likely, intramuscular adipocytes that release adipokines, which lead to insulin resistance, inflammation, and metabolic dysfunction [12, 44], have a negative impact on the overall course of the disease. It is has been described, that myostatosis contributes to insulin abnormalities and diabetes, possibly even independent of obesity [33].
The main limitation of this study is the single center setting with thus limited size and heterogeneity of the patient cohort. Additional investigations with larger patient cohorts are necessary to validate the findings on the potential predictive value of the DECT FF compared also to that of the conventional CT parameters. One would expect that in a larger cohort, not only the change of the DECT FF but also absolute DECT FF values could be defined that predict survival. Theoretically, the predictive value of the DECT FF should at least equal that of the MRA, which has been documented in numerous studies before [17, 45], despite MRA measurements being only semiquantitative and influenced by contrast agent. Concerning the varying time difference between the baseline (CT1) and follow-up CT (CT2), changes of CT parameters were standardized for the respective time interval. Opposed to the SMI and MRA, which were measured based on the whole abdominal muscle as recommended [23] that was not sufficiently feasible for the DECT FF with the syngo.via software. As the muscle status is known to differ between muscle groups [46], in the future, automated segmentation tools should be developed that are compatible with software that allow spectral CT analyses.
Conclusion
In conclusion, the DECT FF, as a new parameter of myosteatosis, appears to be suited to detect increasing muscular fat content in immobilized critically ill patients. Its change was associated with renal failure, the necessity of tracheotomy, and survival. In contrast-enhanced DECT scans, the DECT FF could be a more robust imaging biomarker of muscular fat contents than the MRA. It has the potential for predictive models on morbidity and mortality, further body composition studies, and clinical use, e.g., for risk stratification and informing treatment decisions on nutritional regimes and physiotherapy.
Abbreviations
- 95% CI:
-
95% confidence interval
- BIA:
-
Bioelectrical impedance analysis
- BMI:
-
Body mass index
- CCI:
-
Charlson Comorbidity Index
- CT:
-
Computed tomography
- DE:
-
Dual-energy
- FF:
-
Fat fraction
- HR:
-
Hazard ratio
- ICU:
-
Intensive care unit
- MRA:
-
Muscle radiodensity attenuation (HU)
- MRI:
-
Magnetic resonance imaging
- SAPS II:
-
Simplified Acute Physiology Score II
- SAT:
-
Subcutaneous adipose tissue area (cm2)
- SMI:
-
Skeletal muscle index (cm2/m2)
- SOFA:
-
Sequential Organ Failure Assessment
- VAT:
-
Visceral adipose tissue area (cm2)
- WC:
-
Waist circumference (cm)
References
Peterson SJ, Braunschweig CA (2016) Prevalence of sarcopenia and associated outcomes in the clinical setting. Nutr Clin Pract 31:40–48
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:601
Kizilarslanoglu MC, Kuyumcu ME, Yesil Y, Halil M (2016) Sarcopenia in critically ill patients. J Anesth 30:884–890
Yuan S, Larsson SC (2023) Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism 144:155533
Coletta G, Phillips SM (2023) An elusive consensus definition of sarcopenia impedes research and clinical treatment: a narrative review. Ageing Res Rev 86:101883
Engel HJ, Needham DM, Morris PE, Gropper MA (2013) ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med 41:S69–S80
Akan B (2021) Influence of sarcopenia focused on critically ill patients. Acute Crit Care 36:15–21
Witteveen E, Sommers J, Wieske L et al (2017) Diagnostic accuracy of quantitative neuromuscular ultrasound for the diagnosis of intensive care unit-acquired weakness: a cross-sectional observational study. Ann Intensive Care 7:40
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Zamboni M, Gattazzo S, Rossi AP (2019) Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med 10:5–6
Li CW, Yu K, Shyh-Chang N et al (2022) Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle 13:781–794
Ahn H, Kim DW, Ko Y et al (2021) Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: a new paradigm beyond sarcopenia. Ageing Res Rev 70:101398
Penton CM, Thomas-Ahner JM, Johnson EK, McAllister C, Montanaro F (2013) Muscle side population cells from dystrophic or injured muscle adopt a fibro-adipogenic fate. PLoS One 8:e54553
Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR (2020) Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 145:102839
Poltronieri TS, de Paula NS, Chaves GV (2020) Assessing skeletal muscle radiodensity by computed tomography: an integrative review of the applied methodologies. Clin Physiol Funct Imaging 40:207–223
Somerson JS, Hsu JE, Gorbaty JD, Gee AO (2016) Classifications in brief: goutallier classification of fatty infiltration of the rotator cuff musculature. Clin Orthop Relat Res 474:1328–1332
Aubrey J, Esfandiari N, Baracos VE et al (2014) Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 210:489–497
Molwitz I, Leiderer M, McDonough R et al (2021) Skeletal muscle fat quantification by dual-energy computed tomography in comparison with 3T MR imaging. Eur Radiol 31:7529–7539
Zi Y, Zhang B, Liu L et al (2022) Fat content in lumbar paravertebral muscles: quantitative and qualitative analysis using dual-energy CT in correlation to MR imaging. Eur J Radiol 148:110150
von Elm E, Altman DG, Egger M et al (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808
Hardy EJO, Inns TB, Hatt J et al (2022) The time course of disuse muscle atrophy of the lower limb in health and disease. J Cachexia Sarcopenia Muscle 13:2616–2629
Zopfs D, Theurich S, Grosse Hokamp N et al (2020) Single-slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur Radiol 30:1701–1708
Gomez-Perez S, McKeever L, Sheean P (2020) Tutorial: a step-by-step guide (version 2.0) for measuring abdominal circumference and skeletal muscle from a single cross-sectional computed-tomography image using the national institutes of health imagej. JPEN J Parenter Enteral Nutr 44:419–424
Molwitz I, Leiderer M, Ozden C, Yamamura J (2020) Dual-energy computed tomography for fat quantification in the liver and bone marrow: a literature review. Rofo 192:1137–1153
Molwitz I, Campbell GM, Yamamura J et al (2022) Fat quantification in dual-layer detector spectral computed tomography: experimental development and first in-patient validation. Invest Radiol 57:463–469
Gassenmaier S, Kahm K, Walter SS, Machann J, Nikolaou K, Bongers MN (2021) Quantification of liver and muscular fat using contrast-enhanced Dual Source Dual Energy Computed Tomography compared to an established multi-echo Dixon MRI sequence. Eur J Radiol 142:109845
Rahemi H, Nigam N, Wakeling JM (2015) The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. J R Soc Interface 12:20150365
Callahan LA, Supinski GS (2009) Sepsis-induced myopathy. Crit Care Med 37:S354–S367
Tiao G, Fagan JM, Samuels N et al (1994) Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest 94:2255–2264
Cox MC, Booth M, Ghita G et al (2021) The impact of sarcopenia and acute muscle mass loss on long-term outcomes in critically ill patients with intra-abdominal sepsis. J Cachexia Sarcopenia Muscle 12:1203–1213
Dusseaux MM, Antoun S, Grigioni S et al (2019) Skeletal muscle mass and adipose tissue alteration in critically ill patients. PLoS One 14:e0216991
Hausman GJ, Basu U, Du M, Fernyhough-Culver M, Dodson MV (2014) Intermuscular and intramuscular adipose tissues: Bad vs. good adipose tissues. Adipocyte 3:242–255
Miljkovic I, Zmuda JM (2010) Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care 13:260–264
Nachit M, De Rudder M, Thissen JP et al (2021) Myosteatosis rather than sarcopenia associates with non-alcoholic steatohepatitis in non-alcoholic fatty liver disease preclinical models. J Cachexia Sarcopenia Muscle 12:144–158
Rollins KE, Javanmard-Emamghissi H, Awwad A, Macdonald IA, Fearon KCH, Lobo DN (2017) Body composition measurement using computed tomography: Does the phase of the scan matter? Nutrition 41:37–44
McCollough CH, Leng S, Yu L, Fletcher JG (2015) Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology 276:637–653
Looijaard WGPM, Dekker IM, Stapel SN et al (2016) Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Critical Care 20:386
Moisey LL, Mourtzakis M, Cotton BA et al (2013) Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care 17:R206
Pinto FCS, Andrade MF, Gatti da Silva GH et al (2022) Function over mass: a meta-analysis on the importance of skeletal muscle quality in COVID-19 patients. Front Nutrit 9:837719
van Vugt JLA, Gaspersz MP, Vugts J et al (2019) Low skeletal muscle density is associated with early death in patients with perihilar cholangiocarcinoma regardless of subsequent treatment. Dig Surg 36:144–152
Kemper M, Melling N, Krause L et al (2023) Muscle quality, not quantity, is associated with outcome after colorectal cancer surgery. Eur J Surg Oncol 49:107098
Molwitz I, Kemper M, Krause L et al (2022) Importance of computed tomography muscle quality and continuous versus cut-off-based sarcopenia detection in major hepatic surgery. Ann Transl Med 10:955
Molwitz I, Recklies F, Stark M et al (2023) Muscle quality determined by computed tomography predicts short-term and long-term survival after liver transplantation. Sci Rep 13:7631
Timmers S, Schrauwen P, de Vogel J (2008) Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav 94:242–251
Poltronieri TS, de Paula NS, Chaves GV (2020) Assessing skeletal muscle radiodensity by computed tomography: An integrative review of the applied methodologies. Clin Physiol Funct Imaging 40:207–223
Rollins KE, Gopinath A, Awwad A, Macdonald IA, Lobo DN (2020) Computed tomography-based psoas skeletal muscle area and radiodensity are poor sentinels for whole L3 skeletal muscle values. Clin Nutr 39:2227–2232
Funding
The authors state that this work has not received any funding. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. med. Jennifer Erley, working at University Medical Centre Hamburg-Eppendorf.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Mrs. Ann-Kathrin Ozga from the Institute of Medical Biometry and Epidemiology of the University Medical Centre Hamburg-Eppendorf kindly provided statistical advice for this manuscript and performed most statistical analyses.
Informed consent
Only if the study is on human subjects: Written informed consent was not required for this study because the study design was retrospective, and all CT exams were performed for clinical purposes.
Ethical approval
Institutional Review Board approval was obtained from the local ethics committee (Ärztekammer Hamburg, PV7006-4406-BO-ff).
Study subjects or cohorts overlap
No overlap.
Methodology
-
Retrospective
-
Observational
-
Performed at one institution
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erley, J., Roedl, K., Ozga, AK. et al. Dual-Energy CT muscle fat fraction as a new imaging biomarker of body composition and survival predictor in critically ill patients. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10779-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10779-4