Abstract
Objectives
This study aimed to construct a radiomics-based model for prognosis and benefit prediction of concurrent chemoradiotherapy (CCRT) versus intensity-modulated radiotherapy (IMRT) in locoregionally advanced nasopharyngeal carcinoma (LANPC) following induction chemotherapy (IC).
Materials and methods
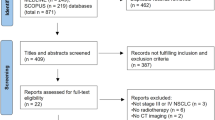
A cohort of 718 LANPC patients treated with IC + IMRT or IC + CCRT were retrospectively enrolled and assigned to a training set (n = 503) and a validation set (n = 215). Radiomic features were extracted from pre-IC and post-IC MRI. After feature selection, a delta-radiomics signature was built with LASSO-Cox regression. A nomogram incorporating independent clinical indicators and the delta-radiomics signature was then developed and evaluated for calibration and discrimination. Risk stratification by the nomogram was evaluated with Kaplan–Meier methods.
Results
The delta-radiomics signature, which comprised 19 selected features, was independently associated with prognosis. The nomogram, composed of the delta-radiomics signature, age, T category, N category, treatment, and pre-treatment EBV DNA, showed great calibration and discrimination with an area under the receiver operator characteristic curve of 0.80 (95% CI 0.75–0.85) and 0.75 (95% CI 0.64–0.85) in the training and validation sets. Risk stratification by the nomogram, excluding the treatment factor, resulted in two groups with distinct overall survival. Significantly better outcomes were observed in the high-risk patients with IC + CCRT compared to those with IC + IMRT, while comparable outcomes between IC + IMRT and IC + CCRT were shown for low-risk patients.
Conclusion
The radiomics-based nomogram can predict prognosis and survival benefits from concurrent chemotherapy for LANPC following IC. Low-risk patients determined by the nomogram may be potential candidates for omitting concurrent chemotherapy during IMRT.
Clinical relevance statement
The radiomics-based nomogram was constructed for risk stratification and patient selection. It can help guide clinical decision-making for patients with locoregionally advanced nasopharyngeal carcinoma following induction chemotherapy, and avoid unnecessary toxicity caused by overtreatment.
Key Points
• The benefits from concurrent chemotherapy remained controversial for locoregionally advanced nasopharyngeal carcinoma following induction chemotherapy.
• Radiomics-based nomogram achieved prognosis and benefits prediction of concurrent chemotherapy.
• Low-risk patients defined by the nomogram were candidates for de-intensification.



Similar content being viewed by others
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- AUC:
-
Area under the receiver operator characteristics curve
- CCRT:
-
Concurrent chemoradiotherapy
- CE-T1WI:
-
Contrast-enhanced T1-weighted images
- CI:
-
Confidence interval
- C-index:
-
Harrell’s concordance index
- CR:
-
Complete response
- DFS:
-
Disease-free survival
- EBV:
-
Epstein–Barr virus
- HR:
-
Hazard ratio
- IC:
-
Induction chemotherapy
- ICC:
-
Inter-class correlation coefficient
- IMRT:
-
Intensity-modulated radiotherapy
- LANPC:
-
Locoregional advanced nasopharyngeal carcinoma
- LASSO:
-
Least absolute shrinkage and selection operator
- MRI:
-
Magnetic resonance imaging
- NPC:
-
Nasopharyngeal carcinoma
- OS:
-
Overall survival
- PACS:
-
Picture archiving and communication system
- PCC:
-
Pearson correlation coefficient
- PD:
-
Disease progression
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RF:
-
Radiomic feature
- ROI:
-
Region of interest
- SD:
-
Stable disease
- T1WI:
-
T1-weighted images
- T2WI:
-
T2-weighted images
References
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J (2019) Nasopharyngeal carcinoma. Lancet 394:64–80
Mao YP, Xie FY, Liu LZ et al (2009) Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys 73:1326–1334
Al-Sarraf M, LeBlanc M, Giri PG et al (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol 16:1310–1317
Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY (2003) Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 21:631–637
Baujat B, Audry H, Bourhis J et al (2006) Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 64:47–56
Blanchard P, Lee A, Marguet S et al (2015) Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 16:645–655
Sun XS, Liu SL, Luo MJ et al (2019) The association between the development of radiation therapy, image technology, and chemotherapy, and the survival of patients with nasopharyngeal carcinoma: a cohort study from 1990 to 2012. Int J Radiat Oncol Biol Phys 105:581–590
Li WF, Li YQ, Chen L et al (2015) Propensity-matched analysis of three different chemotherapy sequences in patients with locoregionally advanced nasopharyngeal carcinoma treated using intensity-modulated radiotherapy. BMC Cancer 15:810
Liu L, Fei Z, Chen M et al (2018) Induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus volumetric modulated arc therapy alone in the treatment of stage II-IVB nasopharyngeal carcinoma patients: a retrospective controlled study. Radiat Oncol 13:148
Wang F, Jiang C, Wang L et al (2020) Influence of concurrent chemotherapy on locoregionally advanced nasopharyngeal carcinoma treated with neoadjuvant chemotherapy plus intensity-modulated radiotherapy: a retrospective matched analysis. Sci Rep 10:2489
Wang Q, Xu G, Xia Y et al (2020) Comparison of induction chemotherapy plus concurrent chemoradiotherapy and induction chemotherapy plus radiotherapy in locally advanced nasopharyngeal carcinoma. Oral Oncol 111:104925
Wei Z, Zhang Z, Luo J, Li N, Peng X (2019) Induction chemotherapy plus IMRT alone versus induction chemotherapy plus IMRT-based concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a retrospective cohort study. J Cancer Res Clin Oncol 145:1857–1864
Lee AW, Tung SY, Chua DT et al (2010) Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 102:1188–1198
Lee AW, Tung SY, Ngan RK et al (2011) Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 47:656–666
Villaflor VM, Melotek JM, Karrison TG et al (2016) Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol 27:908–913
Marur S, Li S, Cmelak AJ et al (2017) E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol 35:490–497
Seiwert TY, Foster CC, Blair EA et al (2019) OPTIMA: a phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann Oncol 30:297–302
Luo WJ, Zou WQ, Liang SB et al (2021) Combining tumor response and personalized risk assessment: potential for adaptation of concurrent chemotherapy in locoregionally advanced nasopharyngeal carcinoma in the intensity-modulated radiotherapy era. Radiother Oncol 155:56–64
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Chen FP, Wen DW, Li F et al (2019) The role of post-neoadjuvant chemotherapy tumor volume for prognostication and treatment guidance in loco-regionally advanced nasopharyngeal carcinoma. Cancers (Basel) 11:1632
Ratain MJ, Eckhardt SG (2004) Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol 22:4442–4445
Limkin EJ, Sun R, Dercle L et al (2017) Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol 28:1191–1206
Keek SA, Leijenaar RT, Jochems A, Woodruff HC (2018) A review on radiomics and the future of theranostics for patient selection in precision medicine. Br J Radiol 91:20170926
Rogers W, Thulasi Seetha S, Refaee TAG et al (2020) Radiomics: from qualitative to quantitative imaging. Br J Radiol 93:20190948
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Gatenby RA, Grove O, Gillies RJ (2013) Quantitative imaging in cancer evolution and ecology. Radiology 269:8–15
Blazic IM, Lilic GB, Gajic MM (2017) Quantitative assessment of rectal cancer response to neoadjuvant combined chemotherapy and radiation therapy: comparison of three methods of positioning region of interest for ADC measurements at diffusion-weighted MR imaging. Radiology 282(2):418–428
Wan L, Peng W, Zou S et al (2021) MRI-based delta-radiomics are predictive of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Acad Radiol Suppl 1:S95–S104
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Saha-Chaudhuri P, Heagerty PJ (2013) Non-parametric estimation of a time-dependent predictive accuracy curve. Biostatistics 14:42–59
Schoenfeld DA (1983) Sample-size formula for the proportional hazards regression model. Biometrics 39:499–503
National Comprehensive Cancer Network (NCCN) (2022) Clinical Practice Guidelines in Oncology. Version 1
Ensley JF, Jacobs JR, Weaver A et al (1984) Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer 54:811–814
Urba S, Wolf G, Eisbruch A et al (2006) Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol 24:593–598
Worden FP, Kumar B, Lee JS et al (2008) Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol 26:3138–3146
Ko CC, Yeh LR, Kuo YT, Chen JH (2021) Imaging biomarkers for evaluating tumor response: RECIST and beyond. Biomark Res 9:52
Peeken JC, Asadpour R, Specht K et al (2021) MRI-based delta-radiomics predicts pathologic complete response in high-grade soft-tissue sarcoma patients treated with neoadjuvant therapy. Radiother Oncol 164:73–82
Chen YP, Liu X, Zhou Q et al (2021) Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet 398:303–313
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10(6):417–427
Teng X, Zhang J, Han X (2023) Explainable machine learning via intra-tumoral radiomics feature mapping for patient stratification in adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Radiol Med 128(7):828–838
Zhang Y, Tang LL, Li YQ, Liu X, Liu Q, Ma J (2019) Spontaneous remission of residual post-therapy plasma Epstein-Barr virus DNA and its prognostic implication in nasopharyngeal carcinoma: a large-scale, big-data intelligence platform-based analysis. Int J Cancer 144:2313–2319
Li W, Chen J, Liang B et al (2021) Long-term monitoring of dynamic changes in plasma EBV DNA for improved prognosis prediction of nasopharyngeal carcinoma. Cancer Med 10:883–894
Funding
This study was supported by the National Natural Science Foundation of China (82272740); and the Sun Yat-Sen University Clinical Research 5010 Program (2020-FXY-406). The funding sources had no role in study design, data collection and analysis, manuscript preparation, or decision to publish.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yan-Ping Mao.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Shun-Xin Wang, Sun Yat-sen University Cancer Center) who has significant statistical expertise performed all statistical analyses. The key raw data underlying this study were uploaded to the Research Data Deposit public platform (RDDA2024775288). Reasonable requests for data sharing should be made to the corresponding author and will be handled in line with the data access and sharing policy of Human Genetic Resource Administration of China and other participating sites outside of China.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained. The institutional ethics committee of Sun Yat-sen University Cancer Center approved this study.
Study subjects or cohorts overlap
The whole study cohort has been reported in our previous study [Luo et al Radiother Oncol 2021], but the difference is that the current study applied radiomics to establish a predictive model and assist clinical decision-making for locoregionally advanced nasopharyngeal carcinoma following induction chemotherapy.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shun-Xin Wang, Yi Yang, and Hui Xie should be considered joint first authors.
Yan-Ping Mao, Li-Zhi Liu, and Yan-Feng Chen should be considered joint senior authors and are co-corresponding authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, SX., Yang, Y., Xie, H. et al. Radiomics-based nomogram guides adaptive de-intensification in locoregionally advanced nasopharyngeal carcinoma following induction chemotherapy. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10678-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10678-8